Cell Signaling
Cell signaling is part of a
complex system of communication that governs basic cellular activities and
coordinates cell actions. The ability of cells to perceive and correctly
respond to their microenvironment is the basis of development, tissue repair,
and immunity as well as normal tissue homeostasis. Errors in cellular
information processing are responsible for diseases such as cancer,
autoimmunity, and diabetes. By understanding cell signaling, diseases may be
treated effectively and, theoretically, artificial tissues may be yielded.
Different types of
signaling:
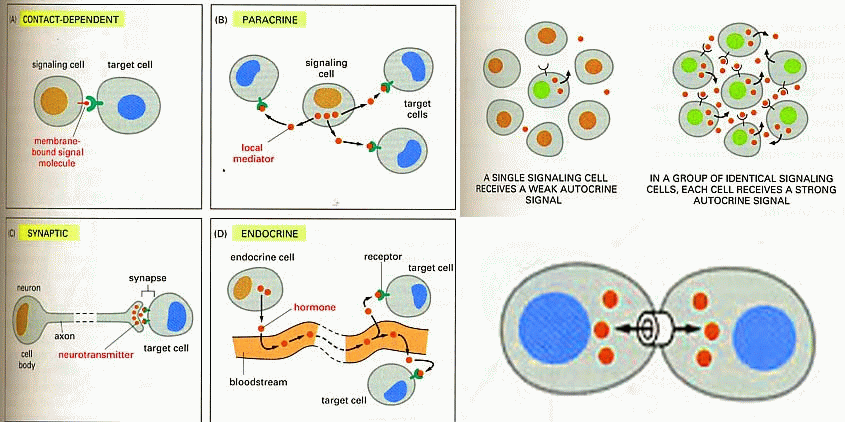
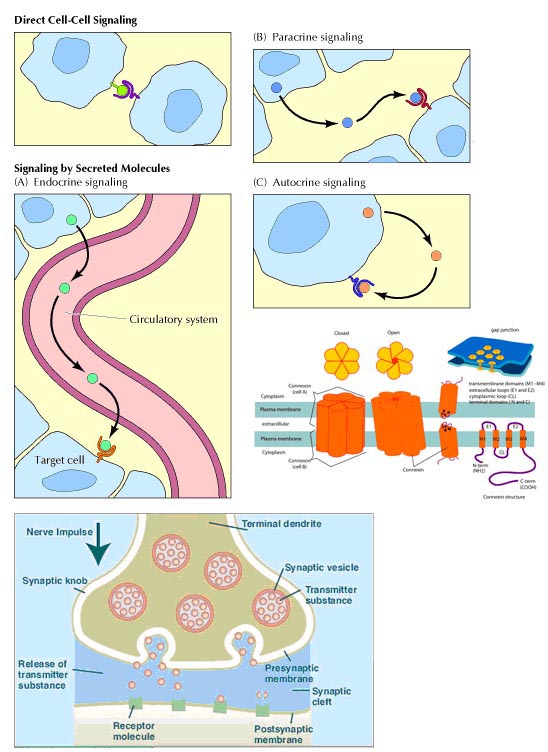
A cell can communicate signals
to other cells in various ways.
Direct signaling is a transfer
of ions or small molecules from one cell to its neighbor through pores in the
membrane. Those pores are built out of membrane proteins and are called gap
junctions. This is the fastest mode of cell-cell communication and is found in
places where extremely fast and well-coordinated activity of cells in needed.
An example of this process can be found in the heart. The muscle cells in the
heart communicate with each other via gap junctions which allow all heart
cells to contract almost simultaneously.
Endocrine signaling utilizes
hormones. A cell secretes chemicals into the bloodstream. Those chemicals
affect the behavior of distant target cells.
Paracrine signaling is a way
for a cell to affect the behavior of neighboring cells by secreting chemicals
into the common intercellular space. This is an important process during
embryonic development.
Autocrine signaling is a way
for a cell to alter its own extracellular environment, which in turn affects
the way the cell functions. The cell secretes chemicals outside of its
membrane and the presence of those chemicals on the outside modifies the
behavior of that same cell. This process is important for growth.
The juxtacrine signaling also
known as contact dependent signaling in which two adjacent cells must make
physical contact in order to communicate. This requirement for direct contact
allows for very precise control of cell differentiation during embryonic
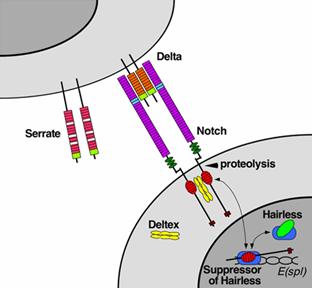
development. Notch signaling is an example for juxtacrine signaling.
Synaptic signaling is found in
the nervous system. It is a highly specific and localized type of paracrine
signalling between two nerve cells or between a nerve cell and a muscle cell.
We will go into details of synaptic signaling when we cover the human nervous
system.
Except autocrine signaling
molecules others actively participate in the intercellular signaling
process. They are also otherwise called as extracellular signaling because
signaling molecule araised from extracellular region.
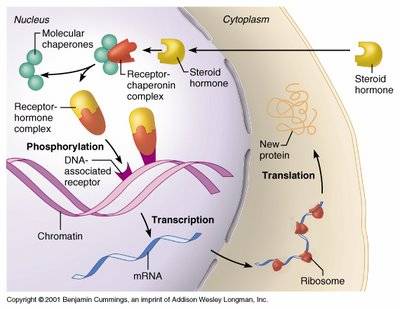
Receptors:
Receptors for cell signaling
mainly are of two types namely cell surface receptors and intracellular or
internal receptors. Those signaling molecules which are capable of diffusing
into cytosol of the cell can interact with internal receptors and execute
signaling process. Steroid molecules and Nitric oxide are examples of
signaling molecules which can bind to internal receptors. They participate in
intracellular signaling process.
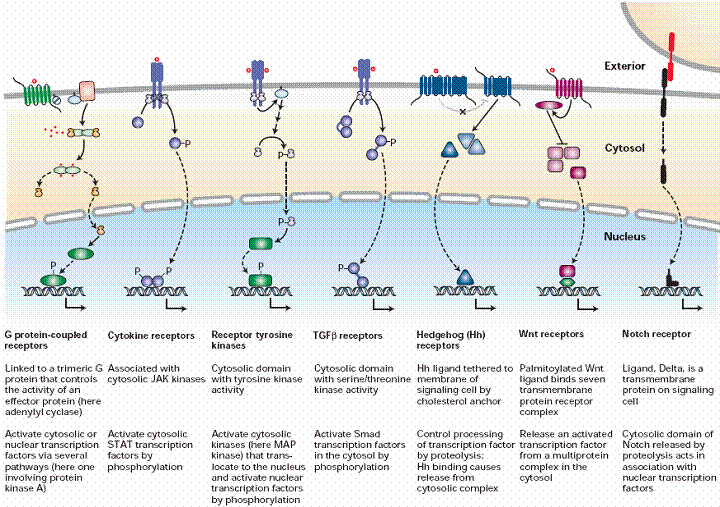
Signaling molecules like
proteins which are unable to enter into cells can interact with the cell
surface receptors and execute its signaling process. Cell surface receptors
are transmembrane proteins whose extracellular portion has the binding site
for the signaling molecule and intracellular portion activates proteins in the
cytosol that in different ways eventually regulate gene transcription in the
nucleus.
Signaling Pathways:
In some cases, receptor
activation caused by ligand binding to a receptor is directly coupled to the
cell's response to the ligand. For example, the neurotransmitter GABA can
activate a cell surface receptor that is part of an ion channel. GABA binding
to a GABA A receptor on a neuron opens a chloride-selective ion channel that
is part of the receptor. GABA A receptor activation allows negatively charged
chloride ions to move into the neuron which inhibits the ability of the neuron
to produce action potentials. However, for many cell surface receptors,
ligand-receptor interactions are not directly linked to the cell's response.
The activated receptor must first interact with other proteins inside the cell
before the ultimate physiological effect of the ligand on the cell's behavior
is produced. Often, the behavior of a chain of several interacting cell
proteins is altered following receptor activation. The entire set of cell
changes induced by receptor activation is called a signal transduction
mechanism or pathway.
In the case of Notch-mediated
signaling, the signal transduction mechanism can be relatively simple.
Activation of Notch can cause the Notch protein to be altered by a protease.
Part of the Notch protein is released from the cell surface membrane and can
act to change the pattern of gene transcription in the cell nucleus. This
causes the responding cell to make different proteins, resulting in an altered
pattern of cell behavior. Cell signaling research involves studying the
spatial and temporal dynamics of both receptors and the components of
signaling pathways that are activated by receptors in various cell types.
Wnt Pathway:
The name Wnt was coined as a
combination of Wg (wingless) and Int and can be pronounced as 'wint'. The
wingless gene had originally been identified as a segment polarity gene in
Drosophila melanogaster that functions during embryogenesis and also during
adult limb formation during metamorphosis. The INT genes were originally
identified as vertebrate genes near several integration sites of mouse mammary
tumor virus (MMTV). The Int-1 gene and the wingless gene were found to be
homologous, with a common evolutionary origin evidenced by similar amino acid
sequences of their encoded proteins.
The canonical Wnt pathway
describes a series of events that occur when Wnt proteins bind to cell-surface
receptors of the Frizzled family, causing the receptors to activate
Dishevelled family proteins and ultimately resulting in a change in the amount
of β-catenin that reaches the nucleus. Dishevelled (DSH) is a key component of
a membrane-associated Wnt receptor complex which, when activated by Wnt
binding, inhibits a second complex of proteins that includes axin, GSK-3, and
the protein APC. The axin/GSK-3/APC complex normally promotes the proteolytic
degradation of the β-catenin intracellular signaling molecule. After this "β-catenin
destruction complex" is inhibited, a pool of cytoplasmic β-catenin stabilizes,
and some β-catenin is able to enter the nucleus and interact with TCF/LEF
family transcription factors to promote specific gene expression
Hedgehog pathway:
The hedgehog signaling pathway
is one of the key regulators of animal development conserved from flies to
humans. The pathway takes its name from its polypeptide ligand, an
intercellular signaling molecule called Hedgehog (Hh) found in fruit flies of
the genus Drosophila. Hh is one of Drosophila's segment polarity gene
products, involved in establishing the basis of the fly body plan. The
appearance of the stubby and "hairy" larvae inspired the name 'hedgehog' when
the gene mutated.
In mammals, when there is no
hedgehog protein present, the patched (PTC) receptors bind a second
transmembrane protein called smoothened (Smo). However, when Hh protein binds
to patched, the Smo protein separates from Ptc enabling Smo to activate a
zinc-finger transcription factor designated GLI. GLI migrates into the nucleus
when it activates a variety of target genes. Hedgehog signaling plays many
important developmental roles in the animal kingdom. For example, wing
development in Drosophila and development of the brain, GI tract, fingers and
toes in mammals.
Mutations or other sorts of
regulatory errors in the hedgehog pathway are associated with a number of
birth defects as well as some cancers. Basal-cell carcinoma, the most common
skin cancer (and, in fact, the most common of all cancers in much of the
world), usually reveals mutations causing extra-high hedgehog or suppressed
patched activity (both leading to elevated GLI activity).
Cell surface receptors:
Cell surface receptors are
integral membrane proteins and, as such, have regions that contribute to three
basic domains:
Extracellular domains: Some of
the residues exposed to the outside of the cell interact with and bind the
hormone - another term for these regions is the ligand-binding domain.
Transmembrane domains:
Hydrophobic stretches of amino acids are "comfortable" in the lipid bilayer
and serve to anchor the receptor in the membrane.
Cytoplasmic or intracellular
domains: Tails or loops of the receptor that are within the cytoplasm react to
hormone binding by interacting in some way with other molecules, leading to
generation of second messengers. Cytoplasmic residues of the receptor are thus
the effector region of the molecule.
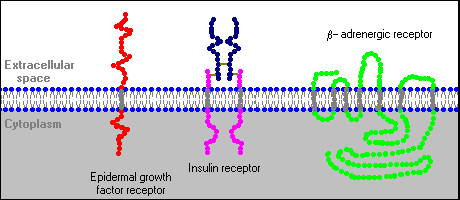
Several distinctive variations
in receptor structure have been identified. As depicted below, some receptors
are simple, single-pass proteins; many growth factor receptors take this form.
Others, such as the receptor for insulin, have more than one subunit. Another
class, which includes the beta-adrenergic receptor, is threaded through the
membrane seven times. Receptor molecules are neither isolated by themselves
nor fixed in one location of the plasma membrane. In some cases, other
integral membrane proteins interact with the receptor to modulate its
activity. Some types of receptors cluster together in the membrane after
binding hormone.
Types of Cell surface receptors:
- G-protein coupled receptors
- Receptor tyrosine kinase receptors
- Cytokine receptors and Non-tyrosine kinase receptors
- Integrin receptors
- Toll-like receptors
- Ligand gated ion-channels receptors
- Receptors with other enzymatic activities
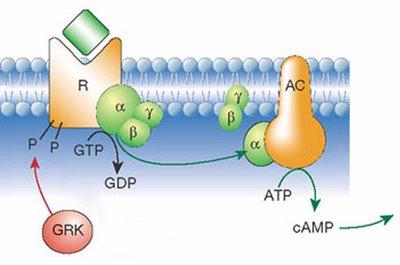
G-protein coupled
receptors:
G protein-linked receptors are
seven-pass transmembrane proteins. This means that the polypeptide chain
traverses the membrane seven times. When a chemical - a hormone or a
pharmaceutical agent - binds to the receptor on the outside of the cell, this
triggers a series of chemical reactions, including the movement and binding of
the G-protein, transformation of GTP into GDP and activation of second
messengers. Second messengers (e.g., cyclic AMP) start a cascade of enzymatic
reactions leading to the cellular response. This signaling method is quite
fast and, more importantly, it amplifies the signal. Binding of a single
hormone molecule quickly results in thousands of molecules of second
messengers acting on even more molecules of enzymes and so on. Thus, the
response to a small stimulus can be very large.
Receptor Tyrosine Kinase:
In contrast to the G
protein-coupled receptors, other cell surface receptors are directly linked to
intracellular enzymes. The largest family of such enzyme-linked receptors are
the receptor protein-tyrosine kinases, which phosphorylate their substrate
proteins on tyrosine residues. This family includes the receptors for most
polypeptide growth factors, so protein-tyrosine phosphorylation has been
particularly well studied as a signaling mechanism involved in the control of
animal cell growth and differentiation. Indeed, the first protein-tyrosine
kinase was discovered in 1980 during studies of the oncogenic proteins of
animal tumor viruses, in particular Rous sarcoma virus, by Tony Hunter and
Bartholomew Sefton. The EGF receptor was then found to function as a
protein-tyrosine kinase by Stanley Cohen and his colleagues, clearly
establishing protein-tyrosine phosphorylation as a key signaling mechanism in
the response of cells to growth factor stimulation.
By now more than 50 receptor
protein-tyrosine kinases have been identified, including the receptors for EGF,
NGF, PDGF, insulin, and many other growth factors. All these receptors share a
common structural organization: an N-terminal extracellular ligand-binding
domain, a single transmembrane α helix, and a cytosolic C-terminal domain with
protein-tyrosine kinase activity. Most of the receptor protein-tyrosine
kinases consist of single polypeptides, although the insulin receptor and some
related receptors are dimers consisting of two pairs of polypeptide chains.
The binding of ligands (e.g., growth factors) to the extracellular domains of
these receptors activates their cytosolic kinase domains, resulting in
phosphorylation of both the receptors themselves and intracellular target
proteins that propagate the signal initiated by growth factor binding.
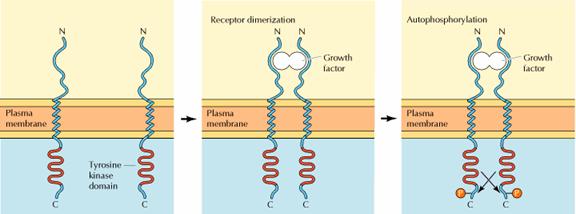
The first step in signaling
from most receptor protein-tyrosine kinases is ligand-induced receptor
dimerization. Some growth factors, such as PDGF and NGF, are themselves dimers
consisting of two identical polypeptide chains; these growth factors directly
induce dimerization by simultaneously binding to two different receptor
molecules. Other growth factors (such as EGF) are monomers but have two
distinct receptor binding sites that serve to crosslink receptors.
Ligand-induced dimerization
then leads to autophosphorylation of the receptor as the dimerized polypeptide
chains cross-phosphorylate one another. Such autophosphorylation plays two key
roles in signaling from these receptors. First, phosphorylation of tyrosine
residues within the catalytic domain may play a regulatory role by increasing
receptor protein kinase activity. Second, phosphorylation of tyrosine residues
outside of the catalytic domain creates specific binding sites for additional
proteins that transmit intracellular signals downstream of the activated
receptors.
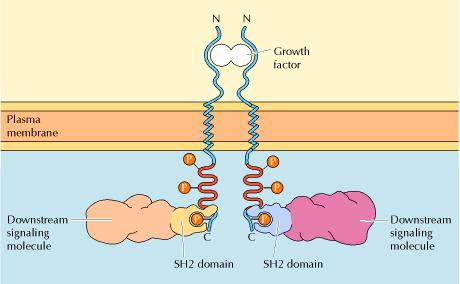
The association of these
downstream signaling molecules with receptor protein-tyrosine kinases is
mediated by protein domains that bind to specific phosphotyrosine-containing
peptides. The best-characterized of these domains are called SH2 domains (for
Src homology 2) because they were first recognized in protein-tyrosine kinases
related to Src, the oncogenic protein of Rous sarcoma virus. SH2 domains
consist of approximately a hundred amino acids and bind to specific short
peptide sequences containing phosphotyrosine residues. The resulting
association of SH2-containing proteins with activated receptor
protein-tyrosine kinases can have several effects: It localizes the
SH2-containing proteins to the plasma membrane, leads to their association
with other proteins, promotes their phosphorylation, and stimulates their
enzymatic activities. The association of these proteins with
autophosphorylated receptors thus represents the first step in the
intracellular transmission of signals initiated by the binding of growth
factors to the cell surface.
Cytokine receptors and
Non-tyrosine kinase receptors:
Rather than possessing
intrinsic enzymatic activity, many receptors act by stimulating intracellular
protein-tyrosine kinases with which they are noncovalently associated. This
family of receptors (called the cytokine receptor superfamily) includes the
receptors for most cytokines (e.g., interleukin-2 and erythropoietin) and for
some polypeptide hormones (e.g., growth hormone). Like receptor
protein-tyrosine kinases, the cytokine receptors contain N-terminal
extracellular ligand-binding domains, single transmembrane α helices, and
C-terminal cytosolic domains. However, the cytosolic domains of the cytokine
receptors are devoid of any known catalytic activity. Instead, the cytokine
receptors function in association with nonreceptor protein-tyrosine kinases,
which are activated as a result of ligand binding.
The first step in signaling
from cytokine receptors is thought to be ligand-induced receptor dimerization
and cross-phosphorylation of the associated nonreceptor protein-tyrosine
kinases. These activated kinases then phosphorylate the receptor, providing
phosphotyrosine-binding sites for the recruitment of downstream signaling
molecules that contain SH2 domains. Combinations of cytokine receptors plus
associated nonreceptor protein-tyrosine kinases thus function analogously to
the receptor protein-tyrosine kinases discussed in the previous section.
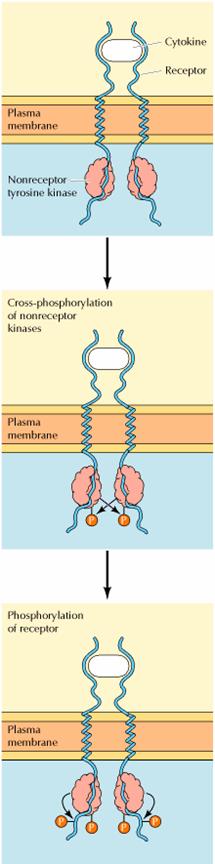
The nonreceptor
protein-tyrosine kinases associated with the cytokine receptors fall into two
major families. Many of these kinases are members of the Src family, which
consists of Src and eight closely related proteins. As already noted, Src was
initially identified as the oncogenic protein of Rous sarcoma virus and was
the first protein shown to possess protein-tyrosine kinase activity, so it has
played a pivotal role in experiments leading to our current understanding of
cell signaling. In addition to Src family members, the cytokine receptors are
associated with nonreceptor protein-tyrosine kinases belonging to the Janus
kinase, or JAK, family. Members of the JAK family appear to be universally
required for signaling from cytokine receptors, indicating that JAK family
kinases play a critical role in coupling these receptors to the tyrosine
phosphorylation of intracellular targets. In contrast, members of the Src
family play key roles in signaling from antigen receptors on B and T
lymphocytes but do not appear to be required for signaling from most cytokine
receptors.
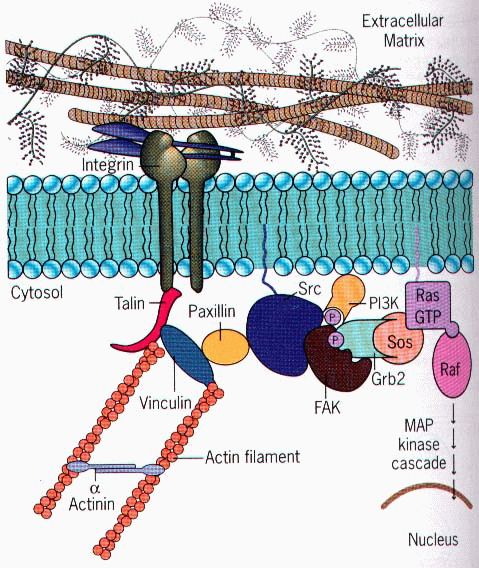
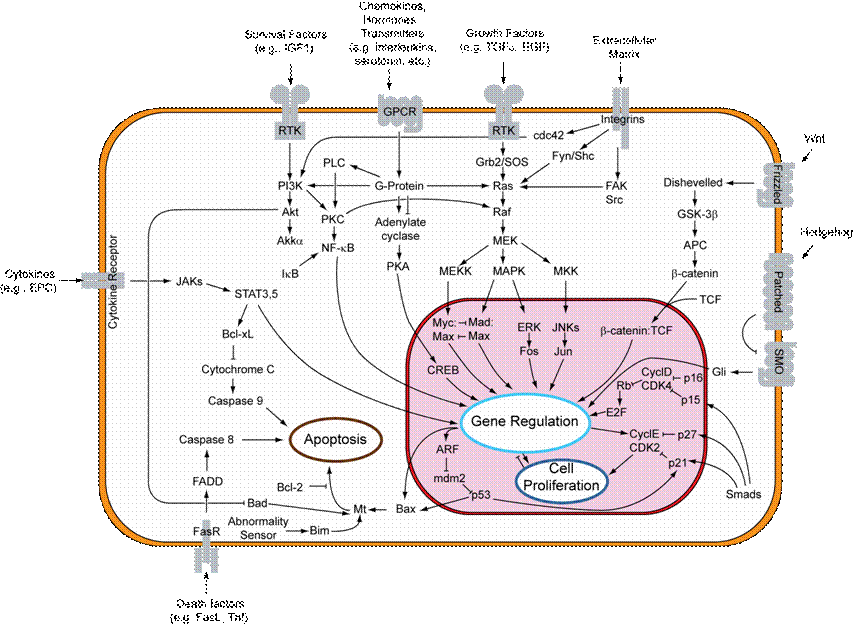
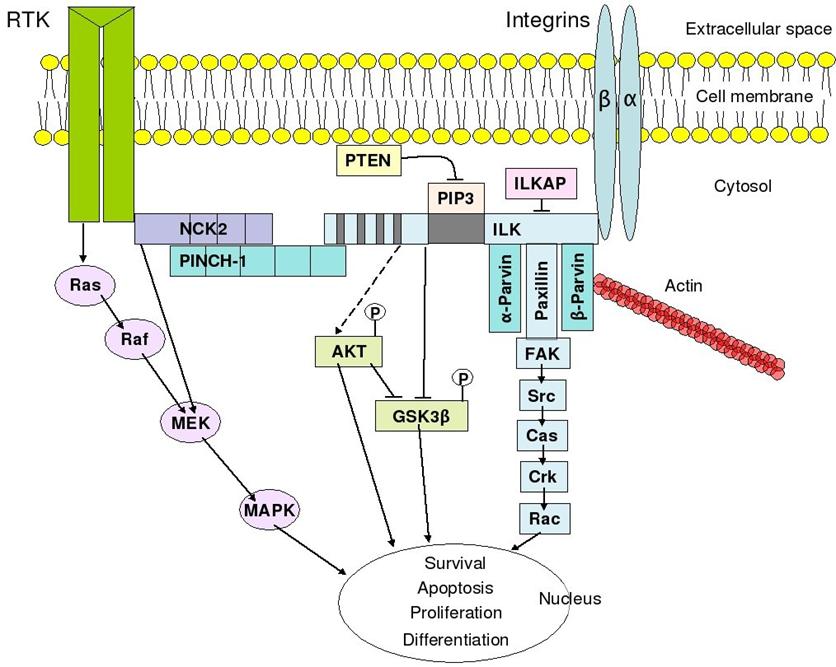
Integrin Receptors:
Integrins are produced by a
wide variety of cell types, and play a role in the attachment of a cell to the
extracellular matrix (ECM) and to other cells, and in the signal transduction
of signals received from extracellular matrix components such as fibronectin,
collagen, and laminin. Ligand-binding to the extracellular domain of integrins
induces a conformational change within the protein and a clustering of the
protein at the cell surface, in order to initiate signal transduction.
Integrins lack kinase activity, and integrin-mediated signal transduction is
achieved through a variety of intracellular protein kinases and adaptor
molecules such as integrin-linked kinase (ILK), focal-adhesion kinase (FAK),
talin, paxillin, parvins, p130Cas, Src-family kinases, and GTPases of the Rho
family, the main protein coordinating signal transduction being ILK. As shown
in the figure, cooperative integrin and receptor tyrosine kinase signaling
determine cellular survival, apoptosis, proliferation, and differentiation.
Important differences exist
between integrin-signaling in circulating blood cells and that in
non-circulating blood cells such as epithelial cells. Integrins at the
cell-surface of circulating cells are inactive under normal physiological
conditions. For example, cell-surface integrins on circulating leukocytes are
maintained in an inactive state in order to avoid epithelial cell attachment.
Only in response to appropriate stimuli are leukocyte integrins converted into
an active form, such as those received at the site of an inflammatory
response. In a similar manner, it is important that integrins at the cell
surface of circulating platelets are kept in an inactive state under normal
conditions, in order to avoid thrombosis. Epithelial cells, in contrast, have
active integrins at their cell surface under normal conditions, which help to
maintain their stable adhesion to underlying stromal cells, which provide
appropriate signals in order to maintain their survival and differentiation.
Toll-like Receptors:
Toll-like receptors (TLRs) are a
class of single membrane-spanning non-catalytic receptors that recognize
structurally conserved molecules derived from microbes once they have breached
physical barriers such as the skin or intestinal tract mucosa, and activate
immune cell responses. They play a key role in the innate immune system. They
receive their name from their similarity to the protein coded by the Toll gene
identified in Drosophila in 1985 by Christiane Nüsslein-Volhard.
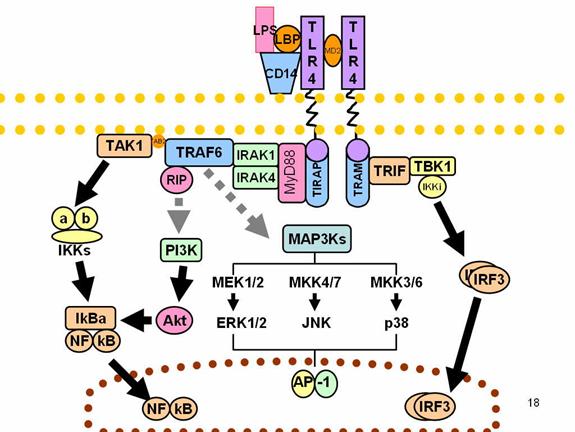
When activated, Toll-like
receptors (TLRs) recruit adapter molecules within the cytoplasm of cells in
order to propagate a signal. Four adapter molecules are known to be involved in
signaling. These proteins are known as MyD88, Tirap (also called Mal), Trif, and
Tram. The adapters activate other molecules within the cell, including certain
protein kinases (IRAK1, IRAK4, TBK1, and IKKi) that amplify the signal, and
ultimately lead to the induction or suppression of genes that orchestrate the
inflammatory response. In all, thousands of genes are activated by TLR
signaling, and, together, the TLRs constitute one of the most powerful and
important gateways for gene modulation.
Following activation by ligands
of microbial origin, several reactions are possible. Immune cells can produce
signalling factors called cytokines which trigger inflammation. In the case of a
bacterial factor, the pathogen might be phagocytosed and digested, and its
antigens presented to CD4+ T cells. In the case of a viral factor,
the infected cell may shut off its protein synthesis and may undergo programmed
cell death (apoptosis). Immune cells that have detected a virus may also release
anti-viral factors such as interferons.
The discovery of the Toll-like
receptors finally identified the innate immune receptors that were responsible
for many of the innate immune functions that had been studied for many years.
Interestingly, TLRs seem only to be involved in the cytokine production and
cellular activation in response to microbes, and do not play a significant role
in the adhesion and phagocytosis of microorganisms.
Ligand gated Ion channels:
When a signaling molecule binds
to an ion channel on the outside of the cell, this triggers the change of
the 3D conformation of the protein and the channel opens, allowing the ions to
move in or out of the cell following their electrical gradients and thus
altering the polarization of the cell membrane. Some ion channels respond to
non-chemical stimuli in the same way, including changes in electrical charge or
mechanical disturbance of the membrane.
Receptors with other enzymatic activity:
Although the vast majority of
enzyme-linked receptors stimulate protein-tyrosine phosphorylation, some
receptors are associated with other enzymatic activities. These receptors
include protein-tyrosine phosphatases, protein-serine/threonine kinases, and
guanylyl cyclases. The functions of most of these receptors are less well
understood than those of either the G protein-coupled receptors or the receptors
associated with protein-tyrosine kinase activity.
Protein-tyrosine phosphatases
remove phosphate groups from phosphotyrosine residues, thus acting to
counterbalance the effects of protein-tyrosine kinases. In many cases,
protein-tyrosine phosphatases play negative regulatory roles in cell signaling
pathways by terminating the signals initiated by protein-tyrosine
phosphorylation. However, some protein-tyrosine phosphatases are cell surface
receptors whose enzymatic activities play a positive role in cell signaling. A
good example is provided by a receptor called CD45, which is expressed on the
surface of T and B lymphocytes. Following antigen stimulation, CD45 is thought
to dephosphorylate a specific phosphotyrosine that inhibits the enzymatic
activity of Src family members. Thus, the CD45 protein-tyrosine phosphatase acts
(somewhat paradoxically) to stimulate nonreceptor protein-tyrosine kinases.
The receptors for transforming
growth factor β (TGF-β) and related polypeptides are protein kinases that
phosphorylate serine or threonine, rather than tyrosine, residues on their
substrate proteins. TGF-β is the prototype of a family of polypeptide growth
factors that control proliferation and differentiation of a variety of cell
types, generally inhibiting proliferation of their target cells. The cloning of
the first receptor for a member of the TGF-β family in 1991 revealed that it is
the prototype of a unique receptor family with a cytosolic protein-serine/threonine
kinase domain. Since then, receptors for additional TGF-β family members have
similarly been found to be protein-serine/threonine kinases. The binding of
ligand to these receptors results in the association of two distinct polypeptide
chains, which are encoded by different members of the TGF-β receptor family, to
form heterodimers in which the receptor kinases cross-phosphorylate one another.
The activated TGF-β receptors then phosphorylate members of a family of
transcription factors called SMADs, which translocate to the nucleus and
stimulate expression of target genes.
Some peptide ligands bind to
receptors whose cytosolic domains are guanylyl cyclases, which catalyze
formation of cyclic GMP. As discussed earlier, nitric oxide also acts by
stimulating guanylyl cyclase, but the target of nitric oxide is an intracellular
enzyme rather than a transmembrane receptor. The receptor guanylyl cyclases have
an extracellular ligand-binding domain, a single transmembrane α helix, and a
cytosolic domain with catalytic activity. Ligand binding stimulates cyclase
activity, leading to the formation of cyclic GMP—a second messenger.
Other receptors bind to
cytoplasmic proteins with additional biochemical activities. For example, the
cytokine tumor necrosis factor (TNF) induces cell death, perhaps as a way of
eliminating damaged or unwanted cells from tissues. The receptors for TNF and
related death-signaling molecules are associated with specific proteases, which
are activated in response to ligand binding. Activation of these
receptor-associated proteases triggers the activation of additional downstream
proteases, ultimately leading to degradation of a variety of intracellular
proteins and death of the cell.
Secondary messenger
In cell physiology, a secondary
messenger system (also known as a second messenger system) is a method of
cellular signaling, whereby a diffusable signaling molecule is rapidly
generated/released which can then go on to activate effector proteins within the
cell to exert a cellular response. Secondary messengers are a component of
signal transduction cascades.
Secondary messenger systems can
be activated by diverse means, either by activation of enzymes that synthesise
them, as is the case with the activation of cyclases which synthesise cyclic
nucleotides, or by opening of ion channels to allow influx of metal ions, such
as in Ca2+ signalling. These small molecules may then go on to exert their
effect by binding to and activating effector molecules such as protein kinases,
ion channels and a variety of other proteins, thus continuing the signalling
cascade.
Types of secondary molecules
There are three basic types of secondary messenger
molecules:
!
Hydrophobic molecules: water-insoluble molecules, like
diacylglycerol, and phosphatidylinositols, which are membrane-associated and
diffuse from the plasma membrane into the juxtamembrane space where they can
reach and regulate membrane-associated effector proteins
!
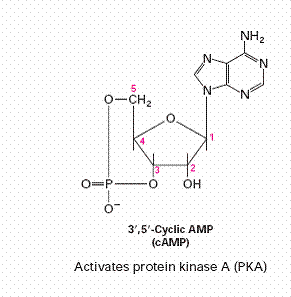
Hydrophilic molecules: water-soluble molecules, like cAMP, cGMP,
IP3, and Ca2+, that are located within the cytosol
!
Gases: nitric oxide (NO) and carbon monoxide (CO), which can
diffuse both through cytosol and across cellular membranes.
These intracellular messengers have some properties in
common:
·
They can be synthesized/released and broken down again in specific
reactions by enzymes or ion channels.
·
Some (like Ca2+) can be stored in special organelles and quickly
released when needed.
·
Their production/release and destruction can be localized,
enabling the cell to limit space and time of signal activity.
Different terminologies used to
differentiate intracellular messengers or molecules namely Primary effector,
Secondary messenger and Secondary effector. Primary effectors include Adenylate
cyclase, Guanylate cyclase, Phospholipase-C, Phospholipase-A and Receptor
tyrosine kinase. Secondary messenger include cAMP, cGMP, IP3 and DAG.
Secondary effector include Protein kinase-A, Protein kinase-G, Protein kinase-C
and Calcium ions.
|
S.No |
Second
Messenger |
Hormones |
Function |
|
1. |
Cyclic AMP |
Epinephrine and nor-epinephrine, glucagon, luteinizing
hormone, follicle stimulating hormone, thyroid-stimulating hormone,
calcitonin, parathyroid hormone, anti-diuretic hormone |
Activates Protein kinase-A |
|
2. |
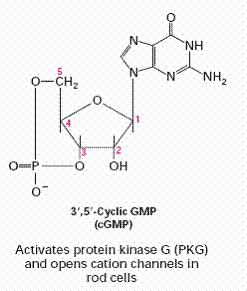
Cyclic GMP |
Atrial naturetic hormone, nitric oxide |
Activates Protein kinase-G and opens cation channels in
rod cells |
|
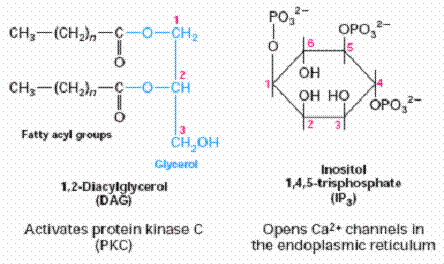
3. |
DAG |
Epinephrine and norepinephrine, angiotensin II,
antidiuretic hormone, gonadotropin-releasing hormone, thyroid-releasing
hormone. |
Activates Protein kinase-C
|
|
4. |
IP3 |
Epinephrine and norepinephrine, angiotensin II,
antidiuretic hormone, gonadotropin-releasing hormone, thyroid-releasing
hormone. |
Opens calcium channels in ER |
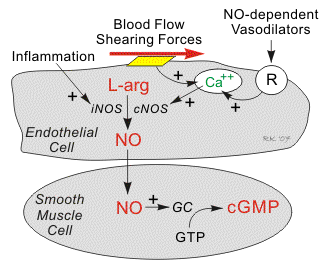
Nitric oxide (NO) as second messenger - The
gas nitric oxide is a free radical that diffuses through the plasma membrane and
affects nearby cells. NO is made from arginine and oxygen by the enzyme NO
synthase, with citrulline as a by-product. NO works mainly through activation of
its target receptor, the enzyme soluble guanylate cyclase, which, when
activated, produces the second messenger cyclic-guanosine monophosphate (cGMP).
NO can also act through covalent modification of proteins or their metal
cofactors. Some of these modifications are reversible and work through a redox
mechanism. In high concentrations, NO is toxic, and is thought to be responsible
for some damage after a stroke. NO serves multiple functions. These include:
- Relaxation of blood vessels
- Regulation of exocytosis of
neurotransmitters
- Cellular immune response
- Modulation of the Hair Cycle
- Production and maintenance
of penile erections
- Activation of apoptosis by
initiating signals that lead to H2AX phosphorylation.