EICOSANOIDS
The eicosanoids consist of the prostaglandins (PGs), thromboxanes (TXs) leukotrienes (LTs) and Lipoxins (LXs). The PGs and TXs are collectively identified as prostanoids. Prostaglandins were originally shown to be synthesized in the prostate gland, thromboxanes from platelets (thrombocytes) and leukotrienes from leukocytes, hence the derivation of their names.

SYNTHESIS OF EICOSANOIDS:
All mammalian cells except erythrocytes synthesize eicosanoids. These molecules are extremely potent, able to cause profound physiological effects at very dilute concentrations. All eicosanoids function locally at the site of synthesis, through receptor-mediated G-protein linked signaling pathways leading to an increase in cAMP levels.
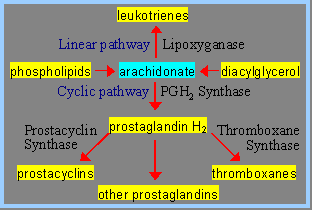
Two main pathways are involved in the biosynthesis of eicosanoids. The prostaglandins and thromboxanes are synthesized by the cyclic pathway, the leukotrienes and lipoxins by the linear pathway.
|
Eicosanoid Abbreviations |
|
|
Prostaglandins
History:
Prostaglandins were first discovered and isolated from human semen in the 1930s by Ulf von Euler of Sweden. Thinking they had come from the prostate gland, he named them prostaglandins. It has since been determined that they exist and are synthesized in virtually every cell of the body except erythrocytes. By then, however, the name prostaglandins was accepted worldwide and hence continued.
Prostaglandins E and F were first isolated from the biological fluids. They were so named due to their solubility in ether and phosphate buffer (fosfat in swedish). All other prostaglandins discovered later were denoted by a letter --> PG A , PG B etc.,
Prostaglandins Structure:
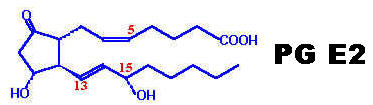
Prostaglandins are unsaturated carboxylic acids, consisting of of a 20 carbon skeleton that also contains a five member ring and are based upon the fatty acid, arachidonic acid. They have a cyclopentane ring which is formed of C8 to C12 and two side chains, with carboxyl group on one side and aliphatic chain on other side. Prostaglandins differ in their structure due to substituent group and double bond on cyclopentane ring. All prostaglandins have OH group at C15 and trans double bond at 13 position. A subscript numeral in the name indicates the number of double bonds in the two side chains. A subscript alpha denotes that the hydroxyl group at that Carbon of the ring and the carboxyl group are on the same side of the ring. Six Prostaglandins of the E and F series namely PG E-1, PG E-2, PG E-3, PG F-1, PG F-2 and PG F-3, are referred to as primary prostaglandins because none is precursor of the other.
RING STRUCTURES:
|
S.No |
Prostaglandin |
Structure |
Substituent group |
|
1. |
PG A |
|
Double bond at C10 -C11 and Keto group at C9 |
|
2. |
PG B |
|
Double bond at C8 - C12 and Keto group C9 |
|
3. |
PG C |
|
Double bond C11 - C12 and Keto group C9 |
|
4. |
PG D |
|
Hydroxyl group at C9 and Keto group at C11 |
|
5. |
PG E |
|
Keto group at C9 and Hydroxyl group at C11 |
|
6. |
PG F |
|
Hydroxyl group at C9 and C11 |
|
7. |
PG G |
|
Two oxygen atoms present at C9 and C11 are bonded and Hydroperoxide present at C15 |
|
8. |
PG H |
|
Two oxygen atoms present at C9 and C11 are bonded and Hydroxyl group at C15 |
|
9. |
PG I |
|
Attachment of oxygen between C6 and C9 forming another five carbon ring |
R- Represents C1 to C7 of prostanoic acid, except in PG I where R is C1 to C5 and R' - Represents C13 to C20 of Prostanoic acid
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Synthesis of Prostaglandins:
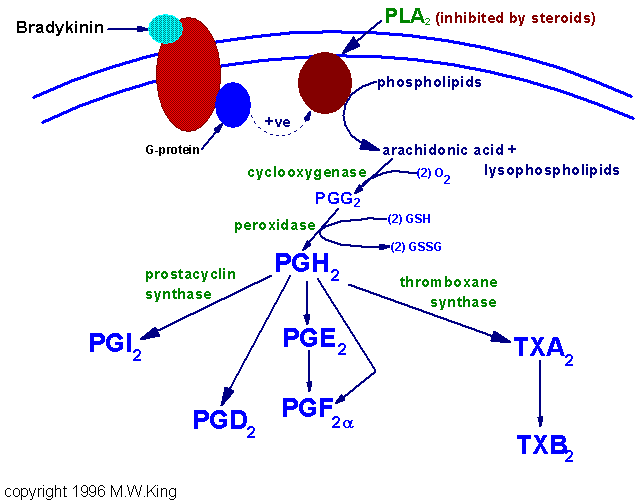
The cyclic pathway is initiated through the action of prostaglandin G/H synthase, PGS (also called prostaglandin endoperoxide synthetase). This enzyme possesses two activities, cyclooxygenase (COX) and peroxidase. There are 2 forms of the COX activity. COX-1 (PGS-1) is expressed constitutively in gastric mucosa, kidney, platelets, and vascular endothelial cells. COX-2 (PGS-2) is inducible and is expressed in macrophages and monocytes in response to inflammation. The primary trigger for COX-2 induction in monocytes and macrophages is platelet-activating factor, PAF and interleukin-1, IL-1. Both COX-1 and COX-2 catalyze the 2-step conversion of arachidonic acid to PGG2 and then to PGH2.
A widely used class of drugs, the non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, indomethacin, naproxen, phenylbutazone and aspirin, all act upon the cyclooxygenase activity, inhibiting both COX-1 and COX-2. Because inhibition of COX-1 activity in the gut is associated with NSAID-induced ulcerations, pharmaceutical companies have developed drugs targeted exclusively against the inducible COX-2 activity [e.g. Celebrex (celecoxib), Bextra (valdecoxib), Prexige (lumiracoxib) and the recently removed Vioxx (rofecoxib)]. Another class, the corticosteroidal drugs, act to inhibit phospholipase A2, thereby inhibiting the release of arachidonate from membrane phospholipids and the subsequent synthesis of eicosinoids.
Catabolism of Prostaglandins:
Prostaglandins are rapidly removed from circulation and metabolized in lungs, brain, liver and tissues. Some 80 to 90% or more is destroyed during a single passage through the Liver or the Lungs. They are degraded by the following steps:
1. Oxidation of secondary alcohol group at C15. This is achieved by a widely distributed prostaglandin specific dehydrogenase called 15-OH prostaglandin dehydrogenase (PGDH). This is the rate limiting step in catabolic pathway.
2. The above is followed by reduction of the double bond at C13. The resulting dihydroderivatives have little or no biological activity.
3. It is followed by beta-oxidation and omega-hydroxylation and oxidation of the side chains.
Mechanism of Action:
The mechanism of action of prostaglandins is not known for certain. They bind to the specific cellular receptors and brings about changes in concentration of "second messenger", which may be cyclic AMP, Ca2+ or even cyclic GMP, which then mediate the biological effects. PG Es act through "cyclic AMP'. PG Fs and Tx-A2 use Ca 2+ as second messenger in some tissues. PG F's action is probably mediated through cyclic GMP as second messenger in some tissues.
Functions of Prostaglandins:
Prostaglandins act as local hormones in their function. They, however, differ from the true hormones in many ways. Prostaglandins are produced in almost all the tissues in contrast to hormonal synthesis which occurs in specialized glands. Prostaglandins are not stored and they are degraded to inactive products at the site of their production. Further, prostaglandins, are produced in very small amounts and have low half lives.
Prostaglandins are involved in a variety of biological functions. The actions of prostaglandins differ in different tissues. Sometimes, prostaglandins bring about opposing actions in the same tissue. Over production of prostaglandins results in many symptoms which include pain, fever, nausea, vomiting etc.,
There are a variety of physiological effects including:
1. Regulation of blood pressure:
The prostaglandins PG E and A are vasodilator in function. This results in increased blood flow and decreased peripheral resistance to lower the blood pressure. Prostaglandins serve as agents in the treatment of hypertension.
2. Inflammation:
The prostaglandins PG E1 and PG E2 induce the symptoms of inflammation like redness, swelling, oedema etc due to arteriolar vasodilation. This led to the belief that prostaglandins are natural mediators of inflammatory reactions of rheumatoid arthritis, psoriasis and conjuctivitis etc. Corticosteroids are frequently used to treat the inflammatory reactions, since they inhibit prostaglandin synthesis.
3. Reproduction:
Prostaglandins have widespread application in the field of reproduction. PG E2 and PG F2 are used for the medical termination of pregnancy and induction of labor. They produce effect by inducing uterine muscle contraction. Thus PG E 2 at rate of 0.5microgram/ml has been used for induction of labour. Same PG in higher doses 5 microgram/ml has been reported to be effective in therapeutic termination of pregnancy which is called as abortifacient.
4. Pain and Fever:
It is believed that pyrogens i.e fever inducing agents, promote prostaglandin biosynthesis leading to the formation of PG E2 in the hypothalamus, the site of regulation of body temperature. PG E2 along with histamine and bradykinin cause pain. Migraine is also due to PG E2. Aspirin and other non-steroidal drugs inhibit prostaglandin synthesis and thus control fever and relieve pain.
5. Action in GI tract:
Prostaglandins PG E1, PG E2, and PG A1 inhibit gastric secretion. There is decrease in volume, acid and pepsin content. Action is believed to be exerted directly on secretory cells through cyclic AMP. Based on this, prostaglandins have been used for preventing or alleviating gastric ulcers. Their action is opposite in pancreatic secretion. There is increase in volume, bicarbonate and enzyme content of pancreatic juice. Mucus secretion is also increased in intestine. There is substantial movement of water and electrolytes into intestinal lumen.
6. Immunological Response:
Prostaglandins secreted by macrophages may modulate or decrease the functions of B and T lymphocytes. They may also reduce the proliferation of lymphocytes in response to lymphocyte mitogenic factors.
7. Action in Respiration:
In general PG - Fs contract and PG - Es relax bronchial and tracheal musculatures in various species including man. Thus PG E1 and PG E2 has been used for treatment of asthma.
8. Renal function:
Intravenous infusion of PG E and A produces substantial increase in renal plasma flow (RPF), increase in Glomerular filtration rate (GFR) and increased urinary flow i.e diuresis. This is achieved by decreasing cyclic AMP level. They also increase sodium and potassium excretion and they stimulate renin secretion from JG cells.
9. Effects on Metabolism:
Prostaglandins influence certain metabolic reactions, probably through the mediation of cyclic AMP. PG Es inhibit adenylate cyclase and lowers cyclic AMP level, thus decreasing lipolysis. PG Es have also some insulin like effects on carbohydrate metabolism i.e glycogenesis induction. They exert parathyroidhormone like effects on bone, resulting to mobilization of calcium from bone producing hypercalcemia. They also exerts thyrotropin like effects on thyroid gland.
Effects of Aspirin and other Pain Killers:
Non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin and derivatives of ibuprofen, inhibit Cyclooxygenase activity of PGH2 Synthase. They inhibit formation of prostaglandins involved in fever, pain and inflammation. They inhibit blood clotting by blocking thromboxane formation in blood platelets. Ibuprofen and related compounds act by blocking the hydrophobic channel by which arachidonate enters the Cyclooxygenase active site. An iodinated analog of ibuprofen is seen in the structural diagram above, between the membrane-binding domain and the heme.
Aspirin acetylates a serine residue, near the Cyclooxygenase active site. This prevents binding of arachidonate. The inhibition by aspirin is irreversible. Aspirin blocks an enzyme called cyclooxygenase, COX-1 and COX-2, which is involved with the ring closure and addition of oxygen to arachidonic acid converting to prostaglandins. The acetyl group on aspirin is hydrolyzed and then bonded to the alcohol group of serine as an ester. This has the effect of blocking the channel in the enzyme and arachidonic can not enter the active site of the enzyme. Aspirin is also thought to inhibit the prostaglandin synthesis involved with unwanted blood clotting in coronary heart disease. At the same time an injury while taking aspirin may cause more extensive bleeding.
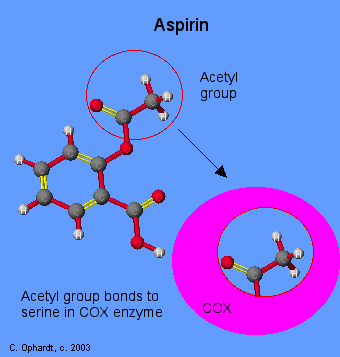
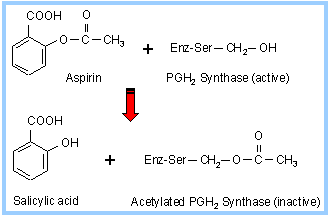
Many people take a daily aspirin for its anti-clotting effect attributed to inhibition of thromboxane formation in blood platelets. The effect is long-lived, because platelets lack a nucleus and do not make new enzyme
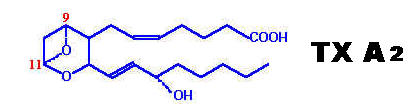
Both prostacyclins and thromboxanes are produced from cyclic endoperoxide PG H2. Both prostacyclins and thromboxanes have a very short half life i.e 5 minutes at 37*C but they are biologically very active. They have powerful effect on contraction of GI smooth muscles, bronchial muscles and umbilical cord vessels. They are antagonistic to each other.
|
S. No |
Prostacyclins (PG I2) |
Thromboxanes (Tx) |
|
1. |
Structure: It contains cyclopentane ring. |
No cyclopentane ring is present but, contains an oxane ring. |
|
2. |
Site of Formation: Principally formed in Vascular endothelium; other sites are Heart and Kidneys. |
Principally formed in platelets; other sites are Neutrophils, Lungs, Brain, Kidney and Spleen. |
|
3. |
Synthesis: Synthesized from cyclic endoperoxide PG -H2 by the enzyme prostacyclin synthase. |
Also synthesized from cyclic endoperoxide PG -H2 by the action of the enzyme Thromboxane synthase. |
|
4. |
Mechanism of Action: Principally by increasing cyclic AMP level in target cell |
Principally by decreasing cyclic AMP level in target cells. Calcium is also used as second messenger in muscle fibers. |
|
5. |
Functions: Principally two main function namely inhibition of platelet aggregation and induces vasodilation which prevent thrombus formation. Other functions include, gastric secretion inhibition, increase renal vasodilation, increase in GFR, increase renin production and relaxes smooth muscles. Ageing, hyperlipaemia and vitamin E deficiency inhibit synthesis of prostacyclins. |
They enhance platelet aggregation and produces vasoconstriction which favors thrombus formation. They also produces smooth muscle contraction, induces release of serotonin, calcium and ADP and does not relax smooth muscles. Imidazoles and dipyridamole inhibit thromboxane synthesis. |
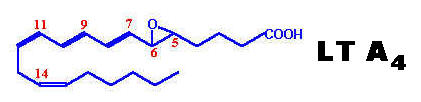
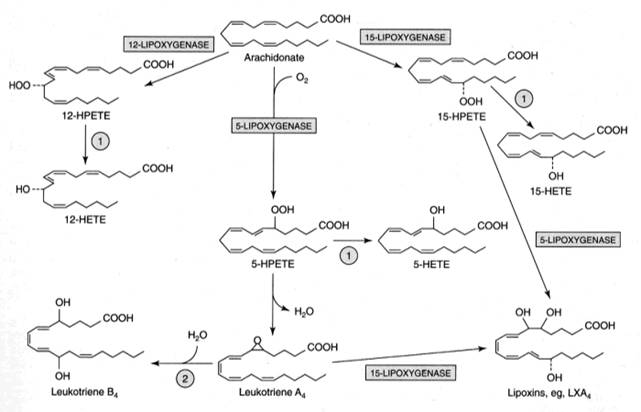
Leukotrienes possess no ring in its structure but have three characteristic double bonds. They are formed from eicosanoic acids in leucocytes, mast cells, and macrophages by the lipo-oxygenase pathway, in response to both immunologic and noninflammatory stimuli. Hydroperoxy eicosa-tetraenoates (HPETE) formed during synthesis of leukotrienes. There are three types of HPETE namely 5-HPETE, 12-HPETE and 15-HPETE. 5-HPETE is the most common and the major product of 5-lipo-oxygenase reaction in polymorphs, basophils, mast cells and macrophages. 12-HPETE is the product of 12-lipo-oxygenase and occurs in platelets, pancreatic endocrine islet cells and glomerular cells of kidney. 15-HPETE occurs in reticulocytes, eosinophils and T-cells. Zileuton, a selective 5-lipo-oxygenase inhibitor inhibits leukotriene synthesis. Numerous stimuli (e.g. epinephrine, thrombin and bradykinin) activate phospholipase A2 which hydrolyzes arachidonic acid from membrane phospholipids. The leukotrienes are identified as LT. The leukotrienes, LTC4, LTD4, LTE4 and LTF4 are known as the peptidoleukotrienes because of the presence of amino acids. The subscript 4 in each molecule refers to the number of -C=C- (double bond) present.
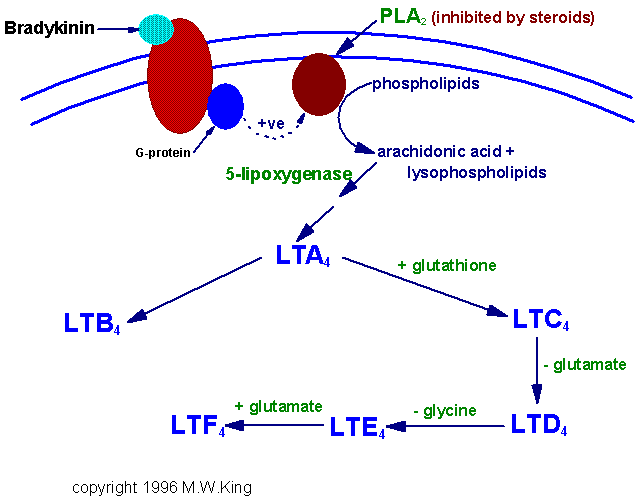
Function of Leukotrienes:
Leukotrienes like LT C4, LT D4 or LT E4 elicits erythma and wheal formation like histamine and also increases vascular permeability. They also induce bronchospasm. LT C4 and LT D4 stimulates potentially mucus secretion from human airway tissue. LT Bs has been found to stimulate chemotaxis and chemokinesis of neutrophils and eosinophils, which are found in large numbers at the site of inflammation.
Mixture of LT C4, LT D4 and LT E4 called as SRS - A (Slow reacting substance of anaphylaxis). Since they are actively involved in anaphylactic reactions, they are called so. These leukotrienes are responsible for intense vasoconstriction of bronchial muscles, vasodilation and increased vascular permeability seen in anaphylactic and allergic reactions.
|
|
|
|
|
|
Lipoxins:
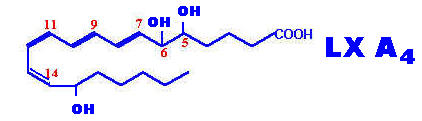
Lipoxins are a family of conjugated tetraenes recently discovered arising in leukocytes by lipo-oxygenase pathway. Several lipoxins have been found to be formed Lx-A4 to Lx-E4 in a manner similar to leukotrienes. They are formed by the combined action of more than one lipo-oxygenase introducing more oxygen into the molecule from arachidonic acid. Lipoxins play role in vasoactive and immunoregulatory function namely act like counter regulatory compounds of immune response.
Properties of Significant Eicosanoids
|
Eicosanoid |
Major site (s) of synthesis |
Major biological activities |
|
PGD2 |
mast cells |
inhibits platelet and leukocyte aggregation, decreases T-cell proliferation and lymphocyte migration and secretion of IL-1a and IL-2; induces vasodilation and production of cAMP |
|
PGE2 |
kidney, spleen, heart |
increases vasodilation and cAMP production, enhancement of the effects of bradykinin and histamine, induction of uterine contractions and of platelet aggregation, maintaining the open passageway of the fetal ductus arteriosus; decreases T-cell proliferation and lymphocyte migration and secretion of IL-1a and IL-2 |
|
PGF2a |
kidney, spleen, heart |
increases vasoconstriction, bronchoconstriction and smooth muscle contraction |
|
PGH2 |
|
precursor to thromboxanes A2 and B2, induction of platelet aggregation and vasoconstriction |
|
PGI2 |
heart, vascular endothelial cells |
inhibits platelet and leukocyte aggregation, decreases T-cell proliferation and lymphocyte migration and secretion of IL-1a and IL-2; induces vasodilation and production of cAMP |
|
TXA2 |
platelets |
induces platelet aggregation, vasoconstriction, lymphocyte proliferation and bronchoconstriction |
|
TXB2 |
platelets |
induces vasoconstriction |
|
LTB4 |
monocytes, basophils, neutrophils, eosinophils, mast cells, epithelial cells |
induces leukocyte chemotaxis and aggregation, vascular permeability, T-cell proliferation and secretion of INF-g, IL-1 and IL-2 |
|
LTC4 |
monocytes and alveolar macrophages, basophils, eosinophils, mast cells, epithelial cells |
component of SRS-A, microvascular vasoconstrictor, vascular permeability and bronchoconstriction and secretion of INF-g |
|
LTD4 |
monocytes and alveolar macrophages, eosinophils, mast cells, epithelial cells |
predominant component of SRS-A, microvascular vasoconstrictor, vascular permeability and bronchoconstriction and secretion of INF-g |
|
LTE4 |
mast cells and basophils |
component of SRS-A, microvascular vasoconstrictor and bronchoconstriction |
**SRS-A = slow-reactive substance of anaphylaxis
ALCOHOLS:
Alcohols found in lipid molecules may be saturated. These commonly include glycerol, cholesterol and higher alcohols such as cetyl alcohol and myricyl alcohol.
CH3(CH2)14CH2OH -- CETYL ALCOHOL CH3(CH2)28CH2OH -- MYRICYL ALCOHOL
Among the unsaturated alcohols found in fats are included a number of pigments like phytol and lycophyll. The former is a constituent of chlorophyll whereas the latter is found in tomatoes as a purple pigment
GLYCEROL:
Glycerol is commonly known as glycerin. It is the simplest trihydric alcohol as it contains three hydroxyl groups in the molecule.
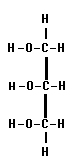
It is colorless oily fluid with a sweetish taste. It is miscible with water and alcohol in all proportions but is almost insoluble in ether. There are two important properties found to be biochemically important they are ester formation and dehydration.
(i) Ester formation:
Glycerol reacts with acids to form mono, di and tri esters.
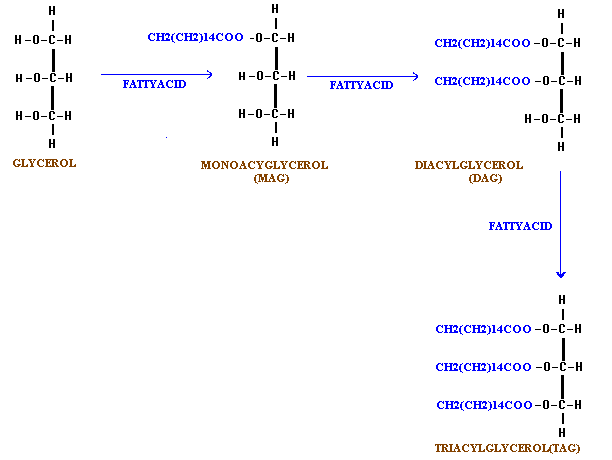
Triesters of glycerol with higher fatty acids constitute fats and oils. Further, the monoglycerides act as good detergents and emulsifying agents because they have a water soluble end i.e the singly esterified glycerol portion and a lipid soluble end i.e the fatty acid portion. This property is considered too be important in the process of digestion of fats and led to the development of synthesis detergents which do not form insoluble substances with the calcium or magnesium ions of hard water.
(ii) Dehydration:
When glycerol is heated in the presence of dehydrating agent like sulphuric acid, phosphorus pentoxide and potassium hydrogen sulphate, it produces an unsaturated aldehyde known as acrylic aldehyde or acrolein.
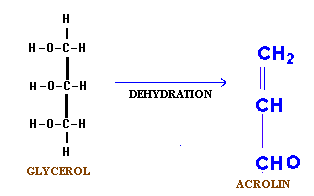
Acrolin has the characteristic unpleasant odour associated with the burning of fats. This reaction occurs whether glycerol is in the free or combined state. It is also used to test the presence of glycerol.
Source:
In industrial side, it is obtained as a by product of soap manufacture. It is also obtainable in the fermentation of glucose by changing conditions in such a way as to decrease the formation of CO2 and alcohol. In physiological state, dietary source act as exogenous source while lipolysis of fats in adipose tissue act as endogenous source for glycerol.
Uses of glycerol:
1. In Industry: Many pharmaceuticals and cosmetic preparations have glycerol in their formulas. This is because of its solubility, it solvent actin and its hygroscopic nature.
2. In Medicine: Nitroglycerine is used as a vasodilator. Glycerol therapy in cerebrovascular diseases reduces cerebral oedema.
3. In Physiology: In body, glycerol has a definite nutritive value. It can be converted to glucose and glycogen, the process called as gluconeogenesis.
STEROL:
Sterols, such as cholesterol, are alcohols with the cyclopentanophenanthrene ring system (atoms 1 through 17 in the structure below). This substructure is also found in steroid hormones such as testosterone, progesterone, and cortisol. Cholesterol is classified as an alcohol because it has a hydroxyl group (-OH) in position 3 of the ring system. Cholesterol is produced by the liver and is found in all body tissues where it helps to organize cell membranes and control their permeability. Cholesterol derivatives in the skin are converted to vitamin D when the skin is exposed to sunlight. Vitamin D3 mediates intestinal calcium absorption and bone calcium metabolism. A high level of cholesterol in the blood is considered to be a risk factor for cardiovascular diseases. Cholesterol and lipoprotein levels can be normalized through exercise and reduced Calorie diets that eliminate hydrogenated fats and add sources of linoleic acid such as grape seed oil.
|
|
|
|
|
Cholesterol |
Vitamin D3 |
Testosterone |
Sterols of vegetable origin are called "phytosterols". They have the same basic structure as cholesterol, but differ in the side chains attached to carbon 17. Phytosterols, such as stigmasterol from soybean oil, are of current interest because they lower blood cholesterol levels. Sterols that are fully saturated (no double bonds) are called "stanols". For example, stigmastanol has the same structure as stigmasterol, but without the double bonds. When fatty acids react with the hydroxyl at carbon 3 they form "sterol esters".
|
|
|
Stigmasterol (a phytosterol) |
CHOLESTEROL:
Cholesterol is an extremely important biological molecule that has roles in membrane structure as well as being a precursor for the synthesis of the steroid hormones and bile acids. Both dietary cholesterol and that synthesized de novo are transported through the circulation in lipoprotein particles. The same is true of cholesteryl esters, the form in which cholesterol is stored in cells. The synthesis and utilization of cholesterol must be tightly regulated in order to prevent over-accumulation and abnormal deposition within the body. Of particular importance clinically is the abnormal deposition of cholesterol and cholesterol-rich lipoproteins in the coronary arteries. Such deposition, eventually leading to atherosclerosis, is the leading contributory factor in diseases of the coronary arteries.
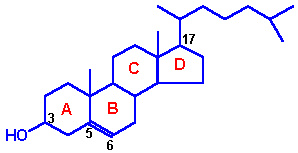
OCCURRENCE:
It is widely present in body tissues. Cholesterol is found in largest amounts in normal human adults brain and nervous tissue 2%, in the liver about 0.3% , skin 0.3% and intestinal mucosa 0.2% , certain endocrine glands example adrenal cortex contain some 10% or more, corpus luteum is also rich in cholesterol. The relatively high content of cholesterol in skin may be related to vit D formation by U.V. rays and that in the adrenal gland and gonads to steroid hormone synthesis. Cholesterol is present in blood and bile and is usually a major constituent of gall stones.
FORMS OF CHOLESTEROL:
cholesterol occurs both in free form and in ester form, in which it is esterified with fatty acids at -OH group at C3 position. The ester form of cholesterol is also referred as "bound" form. Free cholesterol is equally distributed between plasma and red blood cells, but the latter do not contain esters. In brain and nervous tissue , free form predominates whereas in adrenal cortex it occurs mainly as esterified form. some cholesterol esters are formed in tissues by the transfer of acyl groups from acyl coA to cholesterol by acyl transferase. But most of the plasma cholesterol esters are produced in the plasma itself by the transfer of an acyl group from the beta position of lecithin to cholesterol with the help of the enzyme lecithin cholesterol acyl transferase (LCAT).
Lecithin + cholesterol ========> lysolecithin + cholesterol ester
A genetic deficiency of LCAT produces the disease known as Norum's disease. It is characterised by the increased level of free cholesterol, rise in plasma lecithin and decreased level of lysolecithin and cholesterol ester. Normal level of serum cholesterol in adults is 150 to 250 mg%. Of this normal level 30% present as free cholesterol while remaining amount present as cholesterol esters.
Slightly less than half of the cholesterol in the body derives from biosynthesis de novo. Biosynthesis in the liver accounts for approximately 10%, and in the intestines approximately 15%, of the amount produced each day. Cholesterol synthesis occurs in the cytoplasm and microsomes from the two-carbon acetate group of acetyl-CoA.
The process has five major steps:
1. Acetyl-CoAs are converted to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA)
2. HMG-CoA is converted to mevalonate
3. Mevalonate is converted to the isoprene based molecule, isopentenyl pyrophosphate (IPP), with the concomitant loss of CO2
4. IPP is converted to squalene
5. Squalene is converted to cholesterol.
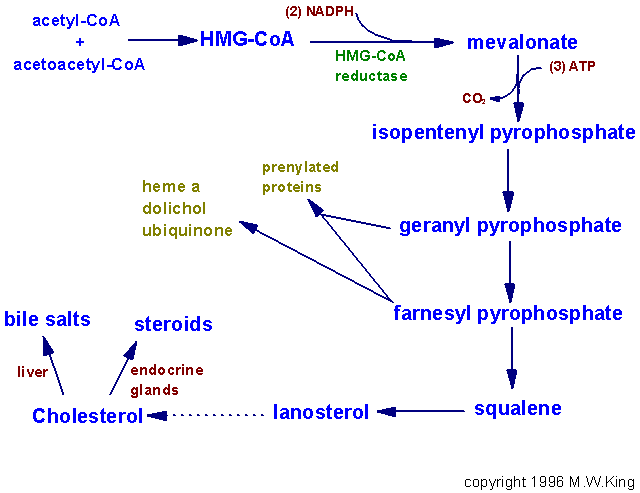
The Utilization of Cholesterol
Cholesterol is transported in the plasma predominantly as cholesteryl esters associated with lipoproteins. Dietary cholesterol is transported from the small intestine to the liver within chylomicrons. Cholesterol synthesized by the liver, as well as any dietary cholesterol in the liver that exceeds hepatic needs, is transported in the serum within LDLs. The liver synthesizes VLDLs and these are converted to LDLs through the action of endothelial cell-associated lipoprotein lipase. Cholesterol found in plasma membranes can be extracted by HDLs and esterified by the HDL-associated enzyme LCAT. The cholesterol acquired from peripheral tissues by HDLs can then be transferred to VLDLs and LDLs via the action of cholesteryl ester transfer protein (apo-D) which is associated with HDLs. Reverse cholesterol transport allows peripheral cholesterol to be returned to the liver in LDLs. Ultimately, cholesterol is excreted in the bile as free cholesterol or as bile salts following conversion to bile acids in the liver.
PROPERTIES OF CHOLESTEROL:
The name "cholesterol" is derived from the Greek word meaning "solid bile". It occurs as a white or faintly yellow, almost odourless, pearly leaflets or granules. It is insoluble in water, sparingly soluble in alcohol and soluble in ether, chloroform, hot alcohol, ethyl acetate and vegetable oils. It easily crystallizes from such solutions in colourless, rhombic plates with one or more characteristic notches in the corner. It is not saponifiable. Its melting point is 147*C to 150*C. Since it has an unsaturated bond, it can take up two halogen atoms.
Reduction of the double bond gives rise to two products corprostanol and cholestanol. Corprostanol occurs in feces and is produced in the intestine as a result of bacterial action on the double bond of cholesterol. The A/B junction is -cis in corprostanol in contrast to -trans in cholesterol. Cholestenol occurs as a minor constituent of the sterols of blood and other tissues. Here the A/B junction in -trans form
COLOR REACTIONS OF CHOLESTEROL:
i)LIBERMANN-BURCHARD REACTION:
A choloform solution of a sterol, when treated with acetic anhydride and concentrated sulphuric acid gives a grass-green color. The usefulness of this reaction is limited by the fact that various sterols give the same or similar colour. This reaction forms the basis for a colorimetric estimation of cholesterol by Sackett's method
ii) SALKOWSKI TEST:
When a chloroform solution of the sterol is treated with an equal volume of concentrated sulphuric acid develops a red to purple colour. The heavier acid which forms a layer below assumes a yellowish colour with a green fluorescence whereas the upper chloroform layer becomes bluish red first and gradually turns violet- red.
iii) ZAK'S METHOD:
When aldehyde free glacial acetic acid solution of cholesterol is treated with ferric chloride and concentrated sulphuric acid, produces a red color. This reaction forms a basis for the colorimetric estimation of cholesterol.
Bile Acids Synthesis and Utilization
The end products of cholesterol utilization are the bile acids, synthesized in the liver. Synthesis of bile acids is one of the predominant mechanisms for the excretion of excess cholesterol. However, the excretion of cholesterol in the form of bile acids is insufficient to compensate for an excess dietary intake of cholesterol. The reaction catalyzed by the 7a-hydroxylase is the rate limiting step in bile acid synthesis.
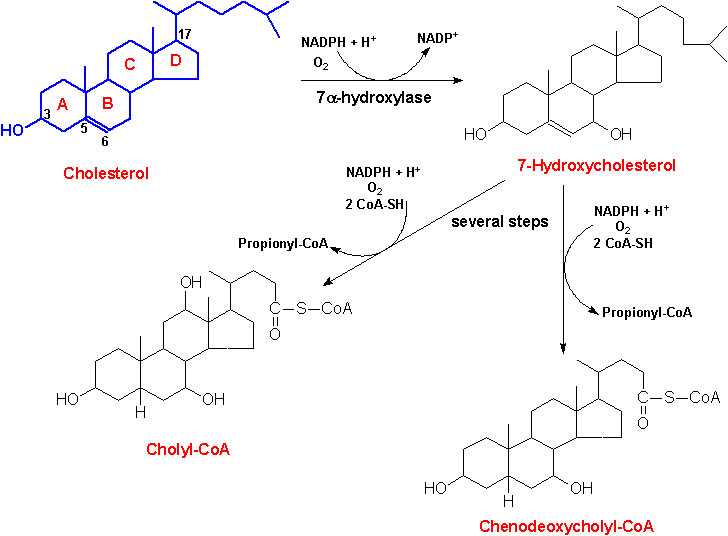
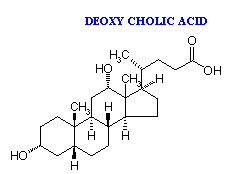
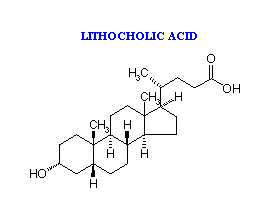
The most abundant bile acids in human bile are chenodeoxycholic acid (45%) and cholic acid (31%). These are referred to as the primary bile acids. Within the intestines the primary bile acids are acted upon by bacteria and converted to the secondary bile acids, identified as deoxycholate (from cholate) and lithocholate (from chenodeoxycholate). Both primary and secondary bile acids are reabsorbed by the intestines and delivered back to the liver via the portal circulation.
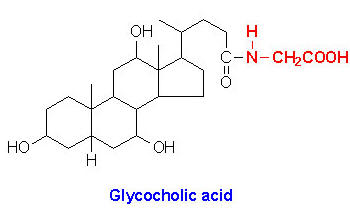
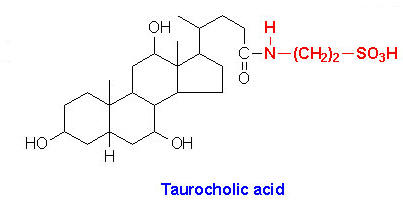
Within the liver the carboxyl group of primary and secondary bile acids is conjugated via an amide bond to either glycine or taurine before their being resecreted into the bile canaliculi. These conjugation reactions yield glycoconjugates and tauroconjugates, respectively. The bile canaliculi join with the bile ductules, which then form the bile ducts. Bile acids are carried from the liver through these ducts to the gallbladder, where they are stored for future use. The ultimate fate of bile acids is secretion into the intestine, where they aid in the emulsification of dietary lipids. In the gut the glycine and taurine residues are removed and the bile acids are either excreted (only a small percentage) or reabsorbed by the gut and returned to the liver. This process of secretion from the liver to the gallbladder, to the intestines and finally reabsorbtion is termed the enterohepatic circulation.
Bile acids perform four physiologically significant functions:
· their synthesis and subsequent excretion in the feces represent the only significant mechanism for the elimination of excess cholesterol.
· bile acids and phospholipids solubilize cholesterol in the bile, thereby preventing the precipitation of cholesterol in the gallbladder which finally leads to the condition of gallstone formation.
· they facilitate the digestion of dietary triacylglycerols by acting as emulsifying agents that render fats accessible to pancreatic lipases.
· they facilitate the intestinal absorption of fat-soluble vitamins.
· they also activate the enzyme cholesterol esterase and pancreatic lipase.
STEROID HORMONES:
These include the sex hormones and the hormones from adrenal cortex. These are synthesized in mammals by the corpus luteum, placenta, adrenal cortex, testis and ovary. The activity of sex hormones controlled by the hormones produced by the anterior lobe of hypophysis. Because of this, hormones of the adenohypophysis referred as primary sex hormones and the sex hormones referred as secondary sex hormones.
Steroids hormones can be grouped on the basis of number of carbon present in their structure as follows:
1. Corpus Luteal Hormone -- C 21
2. Adrenal Cortical Hormones -- C 21
3. Testicular Hormones -- C 19
4. Ovarian Hormones -- C 18
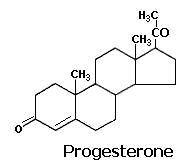
1. Corpus Luteal Hormone -- (Progestrone):
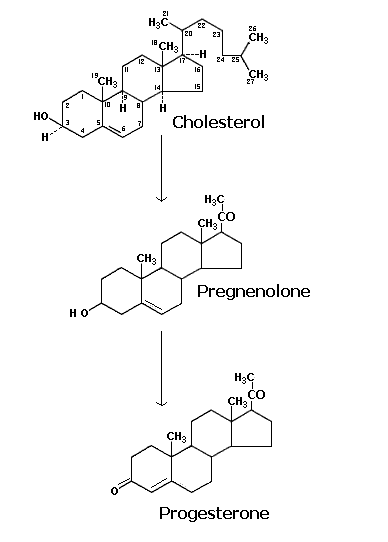
The hormones secreted by the ovarian bed corpus luteum are collectively called as gestogens or progestins. The principal gestogen is progesterone. Progesterone is a C 21 steroid and is secreted by the corpus luteum during the second half of the menstrual cycle. Progesterone is synthesized in the corpus luteum, placenta and the adrenal cortex from its immediate precursor, pregnenolone by a combined dehydrogenase and isomerase reaction. A 20-hydroxy compound, pregnanediol, is the main urinary product of progesterone metabolism in human beings and rabbits.
Functions:
1. The primary function of progesterone is to promote the proliferation of uterine mucosa so that the latter may receive the fertilized ovum. It, thus serves for implantation of the fertilized ovum.
2. It brings mammary glands to full maturity during gestation.
3. It also maintains the uterus quiescent during pregnancy i.e., inhibits contraction of the uterus.
4. If given between 5th and 25th day of the normal menstrual cycle, progesterone exerts an antiovulatory effect. This is the basis for the use of certain progestins as oral contraceptive agents. For example Diosgenin, a compound from Balanites roxburghii wild tree found to be the major ingredient of the contraceptive pill.
5. It serves as precursor of cortisol and corticosterone in the adrenal glands.
2. ADRENAL CORTICAL HORMONES:
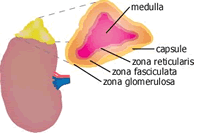
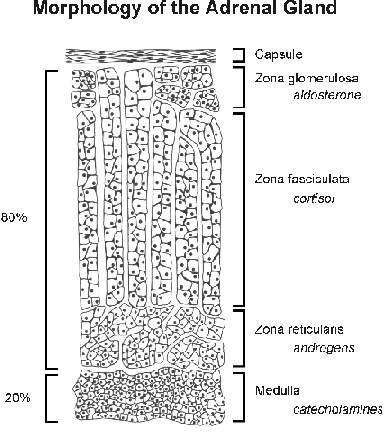
The adrenals or suprarenal glands present on top of kidneys. Each of the two adrenals among mammals has two distinct parts namely an outer covering called the cortex, surrounding an inner dark coloured mass called the medulla. Adrenal cortex is made up of three layers namely:
a. an outer narrow zona glomerulosa, believed to be the site of biosynthesis of the mineralocorticoid hormone.
b. A middle, comparatively broader zona fasciculata, responsible for the production of glucocorticoid hormones and the adrenal androgens.
c. an inner narrow zona reticularis, secreting glucocorticoids along with the middle zone.
Adrenal cortex secretes some 40 - 50 closely related C 21 steroids, collectively called as corticosteroids. Corticosteroids may be grouped under two categories namely mineralocorticoids and glucocorticoids. Mineralocorticoids concerned primarily with the transport of electrolytes and the distribution of water in tissues e.g aldosterone and deoxycorticosterone. Glucocorticoids concered primarily with the metabolism of carbohydrades, protein and fats. e.g. Cortisone, cortisol and corticosterone. Aldosterone is 30 times more active than deoxycorticosterone. Deoxycorticosterone, in its turn, is 4 times more potent than cortisone and and cortisol in maintenance of life. Corticosterone is least active in this regard.
Synthesis:
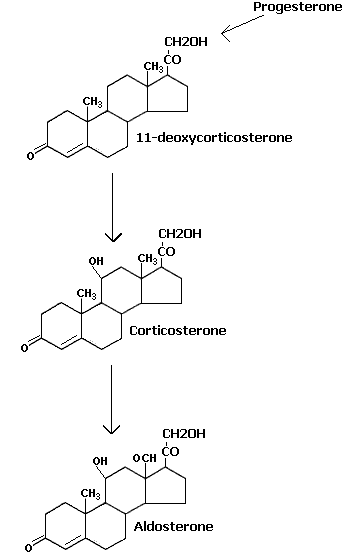
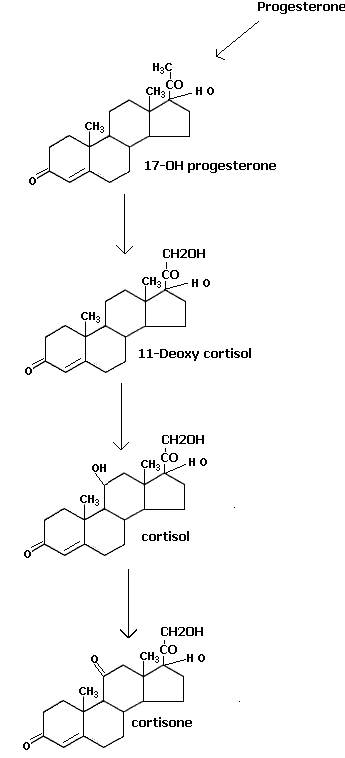
Progesterone act as the substrate to the endoplasmic reticulum 21 hydroxylase enzyme leading to the formation of 11 deoxycorticosterone. It is the immediate precursor of mitochondrial 11-beta hydroxylase leading to the formation of aldosterone through the help of other two enzyme activities namely 18-hydorxy dehydrogenase present in the mitochondria. Glucocorticoids are formed from 17-hydorxy progesterone which inturn is converted by endoplasmic reticulum 21-hydorxylase to 11deoxy cortisol. It is the substrate for 11-beta hydroxylase leading to the formation of cortisol which is the chief glucocorticoid hormone. It is further metabolised into cortisone.
FUNCTIONS:
mineralocorticoids:
Aldosterone is chiefly concerned with water-salt balance of the body. It stimulates the reabsorption of sodium ions from the kidney tubules and as such regulates sodium chloride contents of the blood. This also causes excretion of pottasium in the urine. Aldosterone is also more potent in maintaining the life of adrenalectomized animals.
glucocorticoids:
They influence the carbohydrate metabolism firstly by increasing release of glucose from the liver (glycogenolysis) and secondly by promoting the transformation of amino acids to carbohydrates (gluconeogenesis). They also inhibit protein synthesis in muscle tissues. They also regulate lipogenesis.
Glucocorticoids also decrease immune responses associated with infection and anaphylaxis. The casuse increased secretion of hydrochloric acid and pepsinogen by the stomach and that of trypsinogen by the pancreas. They also cause rention of sodium and water and loss of pottasium to some extent.
Hyperadrenocorticism results in cushing's syndrome whereas hypoadrenocorticism seen addison's disease.
3. TESTICULAR HORMONES:
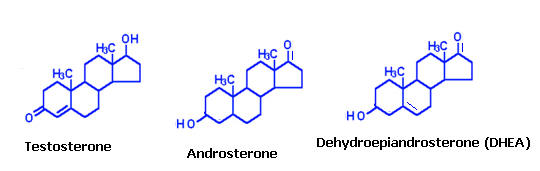
These hormones are secreted mainly by the testes, the male reproductive organs and are called as androgens. Chemically, these are derivatives of a C19 hydrocarbon, androstane. There are many hormones secreted from testes with androgenic activity. The three important ones are testosterone, androsterone and dehydroepiandrosterone. Testosterone is most potent of all these and dehydroepiandrosterone is least active. The relative potency tratio of these three forms is 20:7:1 respectively. Synthesis of testosterone indicated in the following figure:
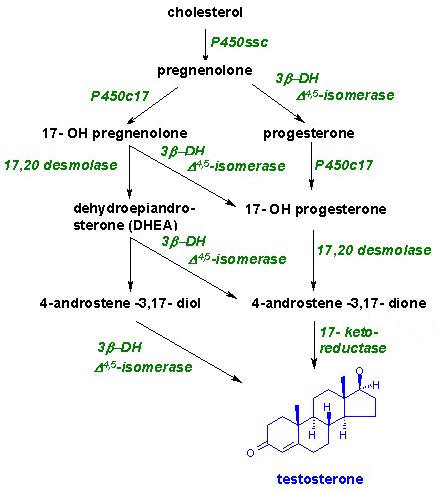
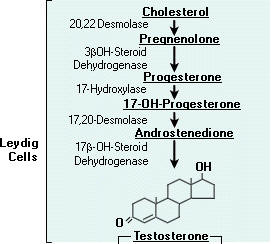
Functions:
The androgens are mainly responsible for the development of secondary sexual characters in male. Androgens regulates the activities of the male reproductive system. The androgens constitute one factor in the production of baldness. Age and inheritance are other factors involved in causing this condition. But baldness does not ensue without androgenic stimulation.
|
S.No |
Characters |
Changes |
|
1. |
Voice |
Larynx enlarges and the vocal cords increase in length and thickness; voice becomes deeper |
|
2. |
Hair growth |
General body hair increases; Hair line on scalp recedes anterolaterally; Hair appear in axillae and around anus; Hair on face grow as beard; |
|
3. |
Menatal |
More aggressive; Active attitude; Interest in opposite sex more pronounced; |
|
4. |
External genitalia |
Penis increases in length and width; scrotum becomes pigmented |
|
5. |
Internal genitalia |
Seminal vesicles enlarge and secrete; the also begin to form fructose; |
|
6. |
Skin |
Sebaceous gland secretion thickness and increases |
|
7. |
Body conformation |
Shoulders broaden and hips remain unaltered, Muscles enlarge, leading to a muscular body contour; |
4. Ovarian Hormones:
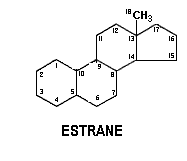
Mammalian ovary contains ovarian follicles and corpus lutea. Hormones produced mainly in the follicles are known as estrogens. Estrogen is a generic term for a substance that induces estrus, which is a cyclic phenomenon of the female reproductive system. Chemically, the estrogens are derivatives of a C18 hydrocarbon estrane.
The three compounds of this group with hormonal activity are estradiol, estriol and estrone. Of all these, beta-estradiol is most potent physiologically, estrone less potent and estriol is least active. Their relative potencies are 50:5:1 respectively. Although ovary is the chief source of estrogens, they are in smaller amounts also produced by the testis and the adrenal cortex.
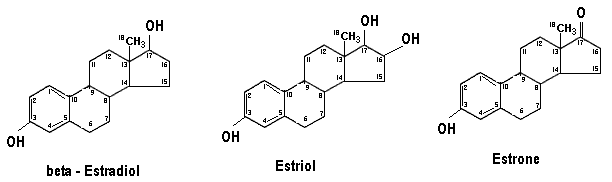
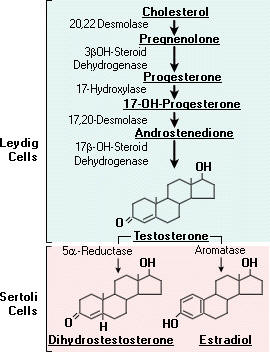
In nonpregnant females, estrogen is mainly synthesised in the ovary. Testosterone is the precursor of estrogens. Estriol is the principal estrogen found in the placenta and urine of pregnant females. It is produced by hydroxylation of estrone at C16 and reduction of keto group at C17.
FUNCTION:
In women, the follicular hormones prepare the uterine mucosa for the later action for the progestational hormones. The changes in the uterine include proliferative growth of the lining of the endometrium, deepening of uterine glands, and increase vascularity; changes in the epithelium of the fallopian tubes and of the vagina also occur. All of these changes begin immediately after menstrual bleeding has ceased.
The estrogens are also effective in the development of secondary sexual characters in females.
|
S.No |
Characters |
Changes |
|
1. |
External Genitalia |
Enlargement of uterus and vagina; Widening of pelvis. |
|
2. |
Internal Genitalia |
Periodic Vaginal bleeding that occurs with the shedding of the uterine mucosa |
|
3. |
Voice |
Larynx retains its prepubertal proportions, i.e., small in size; Voice stays high pitched. |
|
4. |
Hair growth |
Less body hair and more scalp hair; Hair line on scalp resembles that of a child and does not recede anterolaterally. Hair on face absent i.e no beard. |
|
5. |
Mental |
Less aggressive; Passive attitude; Interest in opposite sex less pronunced. |
|
6. |
Body conformation |
Narrow shoulders and broad hips, which are popularly called as 'hips pads'; Thighs that converge and arms that diverge; |
|
7. |
Skin |
Sebaceous gland secretions become more fluid and thus counter the effect of testoserone and inhibit formation of comedones and acne |
Estrogens also influence to a great deal the inorganic metabolism of Calcium and Phosphorous and the organic metabolism of proteins and lipids.
TERPENES:
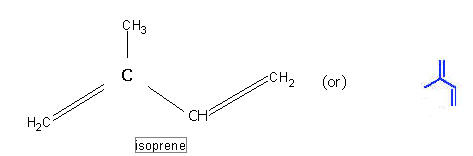
These are the non-saponifiable lipids portion obtain from a number of plants. They are usually long chain compounds derived from a smaller isprene units. Because of this, they are also called as isoprenoids. They are classified as monoterpenes, sesquiterpenes, Diterpenes, Triterpenes and polyterpenes.
In general, these hydrocarbons and their oxygenated derivatives have lesser than 40 carbon atoms.
MONOTERPENES:
The fragrances of many plants arise from volatile terpenes. These are ocur as components of the essential oils, some of which are used as perfumes. Monoterpenes contain molecular formula C10H16 which indicates the presence of two isoprene units. Important monoterpenes are myrcene from oil of bay, geraniol from rose oil, limonene from lemon oil and menthol from peppermint oil.
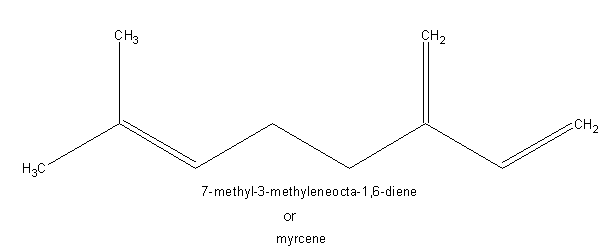
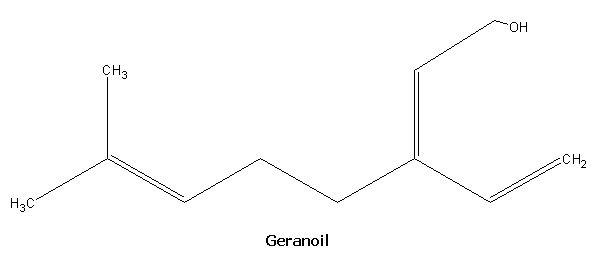
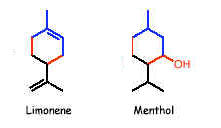
SESQUITERPENES:
Sesquiterpenes contain molecular formula of C15H24. They contain three isoprene units in their structure. Among the sesquiterpenes, farnesol is widely distributed but is present in essential oils in small amounts. It plays vital role in the sythesis of higher terpenes and isprenoid derivatives like cholesterol etc.,

DITERPENES:
They usually contain C20H32 as molecular formula. They contain four isoprene units in their structure. They usually available in both non-cyclic and cyclic forms. Non-cyclic diterpenes include phytol. It is an acyclic diterpene and is obtained from the hydrolysis of chlorophyll. The phytol molecule has 2 chiral centres at 7 and 11 carbon atoms. Natural phytol is very weakly dextrarotatory.
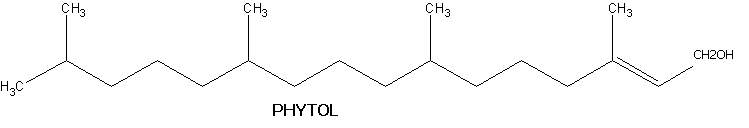
Cyclicditerpenes are of two types namely monocyclic and polycyclic compounds. Example for monocyclic compound is vitamin A1 and vitamin A2.
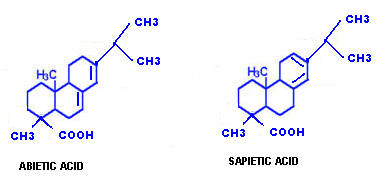
Example for polycyclic diterpenes include abietic and spietic acids.
TRITERPENES:
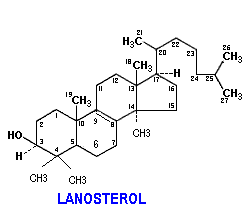
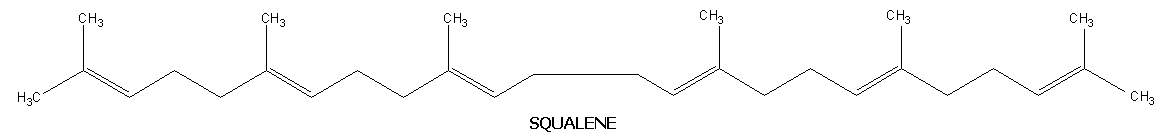
These are not widespread in nature but are significant in that some of them are intermediates in the biosynthesis of cholesterol. They contain molecular formula C30H48 and six isoprene units. Like diterpenes, they are available in cyclic and acyclic forms. Lanosterol and squalene are the examples for cyclic and acyclic triterpenes respectively.
Lanosterol:
Lanosterol plays vital role in biosynthesis of cholesterol. Lanosterol converted into cholesterol in 19 steps. It is the final stage in cholesterol synthesis.
Squqlene:
It is an acyclic hydrocarbon. It was named because it was first isolated from the liver of sharks of genus squalus. It also present in olive oil and leaves of certain plants. Each squalene molecule has 6 double bonds. The double bond system present in squalene is nonconjugated type. It also plays an vital role in the biosynthesis of cholesterol.
POLYSTYRENES:
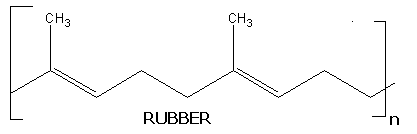
When terpenes contain more than 40 carbons then they are called as polyterpenes. They also present in both cyclic and acyclic forms. Rubber is one of the fine example for acyclic form of polyterpenes. It is present in the latex of many tropical plants. A molecule of rubber is composed of about 500 to 5,000 isoprene units joined in a long straight chain.
CAROTENOIDS:
The carotenoids are tetraterpenes. These are widely distributed in both the plant and animal kingdoms but are exclusively of plant origin. These occur in unsaponifiable residue of plant and animal lipids. They are isoprene derivates with a hgih degree of unsaturation. Because of the presence of many conjugated double bonds, they coloured red or yellow. As an example, the pigment of tomato and that of carrot are red while many oxygen containing carotenoids are yellow. Lycopene, Carotene and Xanthophylls are few biologically significant terpenes.
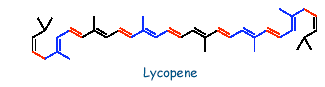
Lycopene:
Lycopene is the main pigment of tomato and paprika. It is a highly unsaturated, unbranched, long chain hydrocarbon and is composed of two identical units(C20H28). A molecule of lycopene consists of 13 double bonds of which 11 are conjugated.
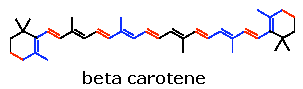
Carotenes:
Another group of naturally occurring carotenoids , with the same molecular formula as that of lycopene, is carotene. There are three types of carotenes namely alpha, beta and gamma carotenes. It is an example for cyclic polyterpenes. In structural comparison, it was concluded that the structure derived by simply closure of lycopene chain ends. Ring closure on only one ends produces gamma carotene while closure of the ring at both ends produces beta and alpha carotenes. Alpha and beta carotenes differ in their position of double bonds in the ring. Of the three types, beta carotene found to be biologically active component because it yields two molecule of vitamin A on hydrolysis.
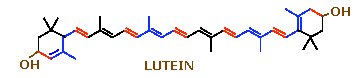
Xanthophylls:
Xanthophylls are characterized by the presence of hydroxyl groups in the ionone rings of carotenes, in para position to the long chain. The xanthophyll of the leaf named as lutein derived from alpha carotene while that of corn which is named as zeaxanthine from beta carotene. The xanthophyll of crustaceans named as astaxanthine is responsible for the appetizing redness of boiled lobsters.
TERPENE DERIVATIVES:
Vitamins like Vitamin K, Vitamin E and Vitamin D are studied under the heading of terpene derivatives. Vitamin K considered as terpene derivative because it contains phytol side chain. Vitamin E considered as terpene derivative because it contains phytol derived side chain in its structure. Vitamin D considered as terpene derivative because it is derived from cholesterol which is synthesized from terpene intermediates.
(Include structure and function of vitamins A, D, E & K)