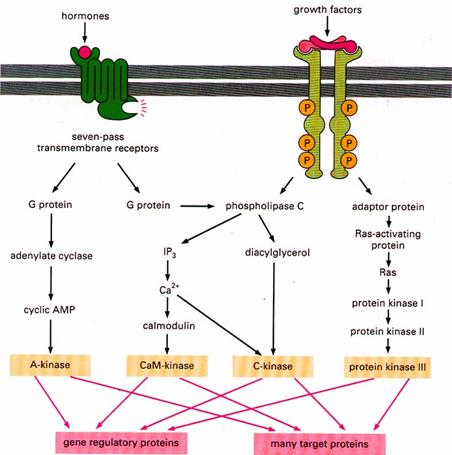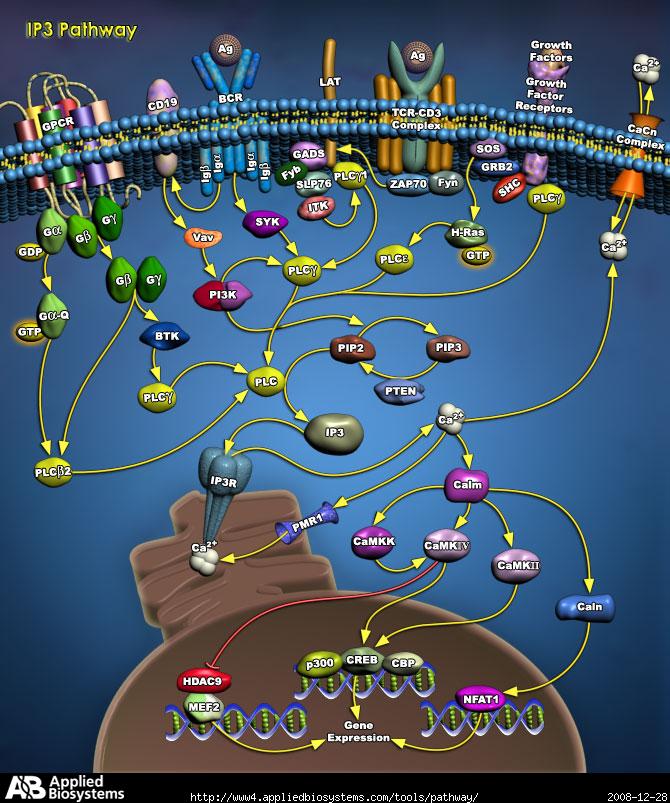
Unit –III: IP3 and DAG Pathway

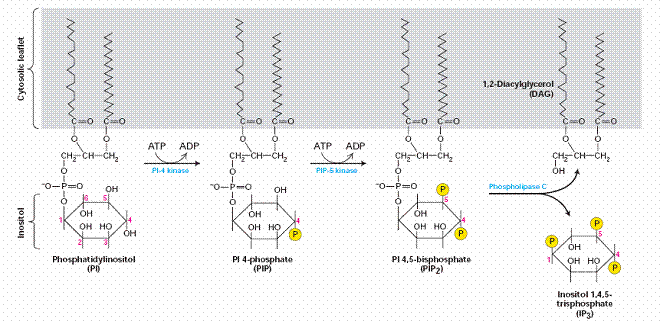
One of the most widespread pathways of intracellular signaling is based on the use of second messengers derived from the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2). PIP2 is a minor component of the plasma membrane, localized to the inner leaflet of the phospholipid bilayer. A number of these second messengers are derived from phosphatidylinositol (PI). The inositol group in this phospholipid, which extends into the cytosol adjacent to the membrane, can be reversibly phosphorylated at several positions by the combined actions of various kinases and phosphatases. These reactions yield several different membrane-bound phosphoinositides.
It is noteworthy that the hydrolysis of PIP2 is activated downstream of both G protein-coupled receptors and protein-tyrosine kinases. This occurs because one form of phospholipase C (PLC-β) is stimulated by G proteins, whereas a second (PLC-γ) contains SH2 domains that mediate its association with activated receptor protein-tyrosine kinases.
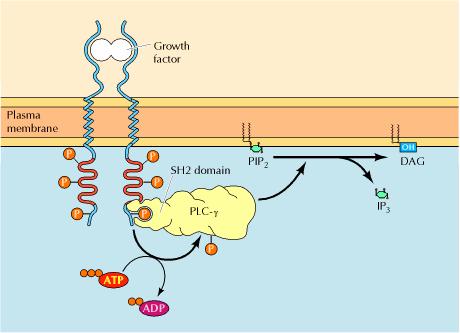
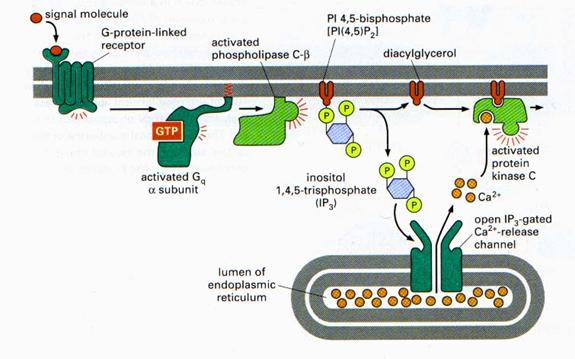
This interaction localizes PLC-γ to the plasma membrane as well as leading to its tyrosine phosphorylation, which increases its catalytic activity. A variety of hormones and growth factors stimulate the hydrolysis of PIP2 by phospholipase C—a reaction that produces two distinct second messengers, diacylglycerol and inositol 1,4,5-trisphosphate (IP3). Diacylglycerol and IP3 stimulate distinct downstream signaling pathways (protein kinase C and Ca2+ mobilization, respectively), so PIP2 hydrolysis triggers a two-armed cascade of intracellular signaling.
After the action of phospholipase-C, the pathway might be studied under two differenet ways namely IP3 pathway and DAG pathway.
IP3 pathway:
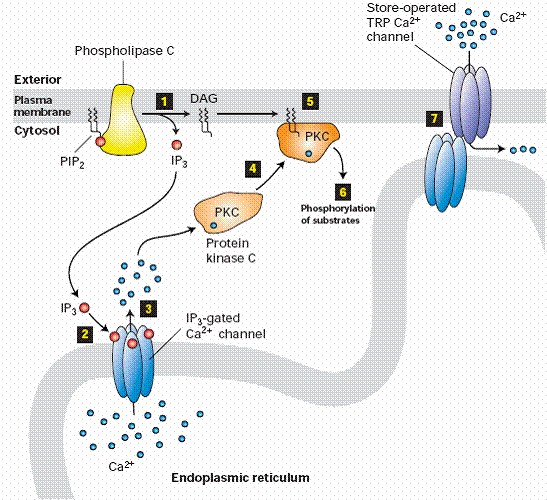
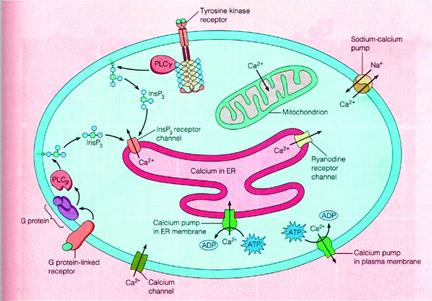
Whereas diacylglycerol remains associated with the plasma membrane, the other second messenger produced by PIP2 cleavage, IP3, is a small polar molecule that is released into the cytosol, where it acts to signal the release of Ca2+ from intracellular stores). The cytosolic concentration of Ca2+ is maintained at an extremely low level (about 0.1 μM) as a result of Ca2+ pumps that actively export Ca2+ from the cell. Ca2+ is pumped not only across the plasma membrane, but also into the endoplasmic reticulum, which therefore serves as an intracellular Ca2+ store. IP3 acts to release Ca2+ from the endoplasmic reticulum by binding to receptors that are ligand-gated Ca2+ channels. As a result, cytosolic Ca2+ levels increase to about 1 μM, which affects the activities of a variety of target proteins, including protein kinases and phosphatases. For example, some members of the protein kinase C family require Ca2+ as well as diacylglycerol for their activation, so these protein kinases are regulated jointly by both arms of the PIP2 signaling pathway.
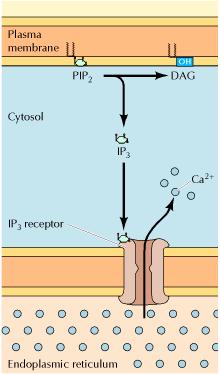
DAG Pathway:
The diacylglycerol produced by hydrolysis of PIP2 activates protein-serine/threonine kinases belonging to the protein kinase C family, many of which play important roles in the control of cell growth and differentiation. A good illustration of this role of protein kinase C is provided by the action of phorbol esters, which have been studied extensively because they promote the growth of tumors in animals. This tumor-promoting activity of the phorbol esters is based on their ability to stimulate protein kinase C by acting as analogs of diacylglycerol. Protein kinase C then activates other intracellular targets, including a cascade of protein kinases known as the MAP kinase pathway, leading to transcription factor phosphorylation, changes in gene expression, and stimulation of cell proliferation. In addition to activating PKC, diacylglycerol has a number of other functions in the cell:
o a source for prostaglandins
o a precursor of the endocannabinoid 2-arachidonoylglycerol
o an activator of a subfamily of TRPC (Transient Receptor Potential Canonical) cation channels, TRPC3/6/7.
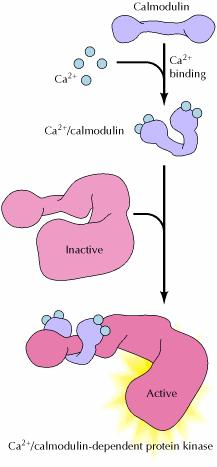
Calcium and calmodulin:
Many of the effects of Ca2+ are mediated by the Ca2+-binding protein calmodulin, which is activated by Ca2+ binding when the concentration of cytosolic Ca2+ increases to about 0.5 μM. Calmodulin (CaM) (an abbreviation for CALcium MODULated proteIN) is a calcium-binding protein expressed in all eukaryotic cells. CaM mediates processes such as inflammation, metabolism, apoptosis, muscle contraction, intracellular movement, short-term and long-term memory, nerve growth and the immune response. It can bind to and regulate a number of different protein targets, thereby affecting many different cellular functions.Ca2+/calmodulin then binds to a variety of target proteins, including protein kinases. One example of such a Ca2+/calmodulin-dependent protein kinase is myosin light-chain kinase, which signals actin-myosin contraction by phosphorylating one of the myosin light chains. Other protein kinases that are activated by Ca2+/calmodulin include members of the CaM kinase family, which phosphorylate a number of different proteins, including metabolic enzymes, ion channels, and transcription factors. One form of CaM kinase is particularly abundant in the nervous system, where it regulates the synthesis and release of neurotransmitters. In addition, CaM kinases can regulate gene expression by phosphorylating transcription factors. Interestingly, one of the transcription factors phosphorylated by CaM kinase is CREB, which (as discussed earlier) is phosphorylated at the same site by protein kinase A. This phosphorylation of CREB illustrates one of many intersections between the Ca2+ and cAMP signaling pathways. Other examples include the regulation of adenylyl cyclases and phosphodiesterases by Ca2+/calmodulin, the regulation of Ca2+ channels by cAMP, and the phosphorylation of a number of target proteins by both protein kinase A and Ca2+/calmodulin-dependent protein kinases. The cAMP and Ca2+ signaling pathways thus function coordinately to regulate many cellular responses.
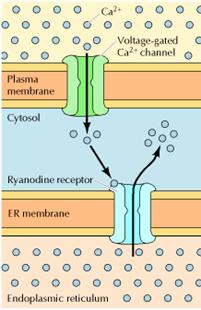
Ca2+ is an extremely common second messenger, and it is important to note that IP3-mediated release of Ca2+ from the endoplasmic reticulum is not the only mechanism by which the intracellular concentration of Ca2+ can be increased. One alternative pathway involves the entry of extracellular Ca2+ through Ca2+ channels in the plasma membrane. In many cells, the transient increase in intracellular Ca2+ resulting from production of IP3 is followed by a more sustained increase resulting from extracellular Ca2+ entry. The entry of extracellular Ca2+ is particularly important in the electrically excitable cells of nerve and muscle, in which voltage-gated Ca2+ channels in the plasma membrane are opened by membrane depolarization). The resulting increases in intracellular Ca2+ then trigger the further release of Ca2+ from intracellular stores by activating distinct Ca2+ channels known as ryanodine receptors. One effect of increases in intracellular Ca2+ in neurons is to trigger the release of neurotransmitters, so Ca2+ plays a critical role in converting electric to chemical signals in the nervous system. In muscle cells, Ca2+ is stored in the sarcoplasmic reticulum, from which it is released by the opening of ryanodine receptors in response to changes in membrane potential. This release of stored Ca2+ leads to large increases in cytosolic Ca2+, which trigger muscle contraction. Cells thus utilize a variety of mechanisms to regulate intracellular Ca2+ levels, making Ca2+ a versatile second messenger that controls a wide range of cellular processes.
Other than diacylglycerol and IP3 messengers:
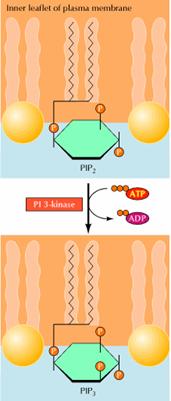
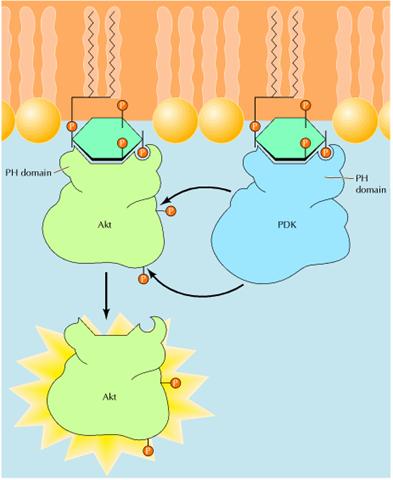
PIP2 not only serves as the source of diacylglycerol and IP3, but is also the starting point of a distinct second messenger pathway that plays a key role in regulating cell survival. In this pathway, PIP2 is phosphorylated on the 3 position of inositol by the enzyme phosphatidylinositide (PI) 3-kinase. Like phospholipase C, one form of PI 3-kinase is activated by G proteins, while a second has SH2 domains and is activated by association with receptor protein-tyrosine kinases. Phosphorylation of PIP2 yields phosphatidylinositol 3,4,5-trisphosphate (PIP3), which functions as a distinct second messenger. A key target of PIP3, which is critical for signaling cell survival, is a protein-serine/threonine kinase called Akt. PIP3 binds to a domain of Akt known as the pleckstrin homology domain. This interaction recruits Akt to the inner face of the plasma membrane, where it is phosphorylated and activated by other protein kinases (called PDKs) that also contain pleckstrin homology domains and bind PIP3. The formation of PIP3 thus results in the association of both Akt and PDKs with the plasma membrane, leading to phosphorylation and activation of Akt. Once activated, Akt phosphorylates a number of target proteins, including proteins that are direct regulators of cell survival, transcription factors, and other protein kinases that regulate cell metabolism and protein synthesis.
Second messengers can also be derived from other phospholipids. The hydrolysis of phosphatidylcholine is stimulated by a variety of growth factors, providing a second source of diacylglycerol, in addition to that derived from PIP2. While PIP2 hydrolysis is a transient response to growth factor stimulation, the hydrolysis of phosphatidylcholine typically persists for several hours, providing a sustained source of diacylglycerol that may be important in signaling long-term responses, such as cell proliferation. Sphingomyelin is also cleaved in response to a variety of extracellular stimuli, resulting in the formation of ceramide. Although its targets remain to be fully elucidated, ceramide regulates a number of protein kinases and phosphatases that can affect cell proliferation and survival.
Protein Kinase – C:
Protein kinase C ('PKC', EC 2.7.11.13) is a family of protein kinases consisting of ~10 isozymes. They are divided into three subfamilies, based on their second messenger requirements: conventional (or classical), novel, and atypical. Conventional (c)PKCs contain the isoforms α, βI, βII, and γ. These require Ca2+, diacylglycerol (DAG), and a phospholipid such as phosphatidylcholine for activation. Novel (n)PKCs include the δ, ε, η, and θ isoforms, and require DAG, but do not require Ca2+ for activation. Thus, conventional and novel PKCs are activated through the same signal transduction pathway as phospholipase C. On the other hand, atypical (a)PKCs (including protein kinase Mζ and ι / λ isoforms) require neither Ca2+ nor diacylglycerol for activation. The term "protein kinase C" usually refers to the entire family of isoforms.
Structure:
The structure of all PKCs consists of a regulatory domain and a catalytic domain tethered together by a hinge region. The catalytic region is highly conserved among the different isoforms, as well as, to a lesser degree, among the catalytic region of other serine/threonine kinases. The second messenger requirement differences in the isoforms are a result of the regulatory region, which are similar within the classes, but differ among them. Most of the crystal structure of the catalytic region of PKC has not been determined, except for PKC theta and iota. Due to its similarity to other kinases whose crystal structure have been determined, the structure can be strongly predicted.
The regulatory domain or the amino-teminus of the PKCs contains several shared subregions. The C1 domain, present in all of the isoforms of PKC has a binding site for DAG as well as non-hydrolysable, non-physiological analogues called phorbol esters. This domain is functional and capable of binding DAG in both conventional and novel isoforms, however, the C1 domain in atypical PKCs is incapable of binding to DAG or phorbol esters. The C2 domain acts as a Ca2+ sensor and is present in both conventional and novel isoforms, but functional as a Ca2+ sensor only in the conventional. The pseudosubstrate region, which is present in all three classes of PKC, is a small sequence of amino acids that mimic a substrate and bind the substrate-binding cavity in the catalytic domain,lack crital serine, threonine phosphoacceptor residues, keeping the enzyme inactive. When Ca2+ and DAG are present in sufficient concentrations, they bind to the C2 and C1 domain, respectively, and recruit PKC to the membrane. This interaction with the membrane results in release of the pseudosubstrate from the catalytic site and activation of the enzyme. In order for these allosteric interactions to occur, however, PKC must first be properly folded and in the correct conformation permissive for catalytic action.
The catalytic region or kinase core of the PKA allows for different functions to be processed, PKB (also known as Akt) and PKC kinases contains approximately 40% amino acid sequence similarity. This similarity increases to ~ 70% across PKCs and even higher when comparing within classes. For example, the two atypical PKC isoforms, ζ and ι/λ, are 84% identical. Of the over-30 protein kinase structures whose crystal structure has been revealed, all have the same basic organization. They are a bilobal structure with a β sheet comprising the N-terminal lobe and an α helix constituting the C-terminal lobe. Both the ATP- and substrate-binding sites are located in the cleft formed by these two lobes. This is also where the pseudosubstrate domain of the regulatory region binds.
Another feature of the PKC catalytic region that is essential to the viability of the kinase is its phosphorylation. The conventional and novel PKCs have three phosphorylation sites, termed: the activation loop, the turn motif, and the hydrophobic motif. The atypical PKCs are phosphorylated only on the activation loop and the turn motif. Phosphorylation of the hydrophobic motif is rendered unnecessary by the presence of a glutamic acid in place of a serine, which, as a negative charge, acts similar in manner to a phosphorylated residue. These phosphorylation events are essential for the activity of the enzyme, and 3-phosphoinositide-dependent protein kinase-1 (PDK1) is the upstream kinase responsible for initiating the process by transphosphorylation of the activation loop.
The consensus sequence of protein kinase C enzymes is similar to that of protein kinase A, since it contains basic amino acids close to the Ser/Thr to be phosphorylated. Their substrates are, e.g., MARCKS proteins, MAP kinase, transcription factor inhibitor IκB, the vitamin D3 receptor VDR, Raf kinase, calpain, and the epidermal growth factor receptor.
Function:
Protein Kinase-C activity promotes smooth muscle contraction, sperm ejaculation, gastric juice secretion, platelet aggregation, csf secretion, H+ secretion, Na+ reabsorption and bronchoconstriction
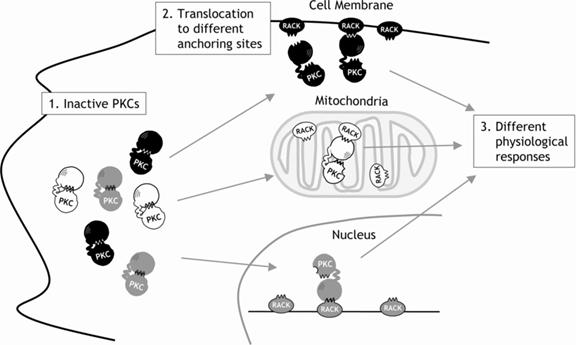
PK-C translocation:
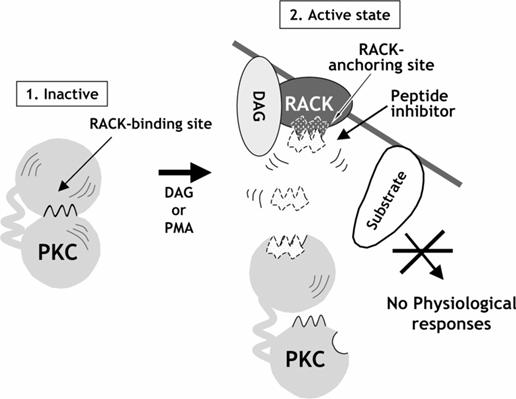
Upon activation, protein kinase C enzymes are translocated to the plasma membrane by RACK proteins (membrane-bound receptor for activated protein kinase C proteins). The protein kinase C enzymes are known for their long-term activation: They remain activated after the original activation signal or the Ca2+-wave is gone. This is presumably achieved by the production of diacylglycerol from phosphatidylcholine by a phospholipase; fatty acids may also play a role in long-term activation. Depending upon their isoenzyme forms, they could bind to different RACK proteins and exhibit its functions in different locations. By blocking the RACK binding region of PK-C, the activity of PK-C might be blocked.
CaM kinases:
Ca2+/calmodulin-dependent protein kinases or CaM kinases (EC 2.7.11.17) are serine/threonine-specific protein kinase are primarily regulated by the Ca2+/calmodulin complex. These kinases show a memory effect on activation.
Two types of CaM kinase are:
Structure:
The CaM kinases consist of an N-terminal catalytic domain, a regulatory domain, and an association domain. The enzymes assemble into dodecameric holoenzyme structures, with the catalytic domains sticking out, such that these may phosphorylate residues in an intersubunit fashion. In the absence of Ca2+/calmodulin, the catalytic domain is autoinhibited by the regulatory domain, which contains a pseudosubstrate sequence. Several CaM kinases aggregate into a homooligomer or heterooligomer. Upon activation by Ca2+/calmodulin, the activated CaM kinases autophosphorylate each other in an intermolecular reaction at the threonine 286 residue. This has two effects:
Phosphorylation at residues 305/306, which are both threonines, have a negative effect on binding of Ca2+/calmodulin complex to enzyme subunits, thus reducing function. The introduction of phospho-mimicking and phospho-null mutations of the enzyme at these sites into mice have shown that both mutations have effects on the way that long term memory is induced.
Function:
Due to its ability for autophosphorylation, CaMK activity can outlast the intracellular calcium transient that is needed to activate it. In neurons, this property is important for the induction of synaptic plasticity. Pharmacological inhibition of CaMKII blocks the induction of long-term potentiation. Upon activation, CaMKII phosphorylates postsynaptic glutamate receptors and thus changes the electrical properties of the synapse.
Example: MLCK
Myosin-light-chain kinase (MLCK) is a serine/threonine-specific protein kinase that phosphorylates the regulatory light chain of myosin II. Three different MLCK isoforms exist. There is a cardiac-MLCK encoded by mylk3, a skeletal-MLCK encoded by mylk2, and smooth muscle-MLCK encoded by mylk. Smooth muscle and non-muscle MLCK are identical and are the product of the same gene, mylk.
This protein is important in the mechanism of contraction in smooth muscle. Once there is an influx of calcium into the smooth muscle, either from the sarcoplasmic reticulum or, more important, from the extracellular space, contraction of smooth muscle fibers may begin. First, the calcium will bind to calmodulin. This binding will activate MLCK, which will go on to phosphorylate the myosin light chain at serine residue 19. This will enable the myosin crossbridge to bind to the actin filament and allow contraction to begin (through the crossbridge cycle). Since smooth muscle does not contain a troponin complex like striated muscle does, this mechanism is the main pathway for regulating smooth muscle contraction.
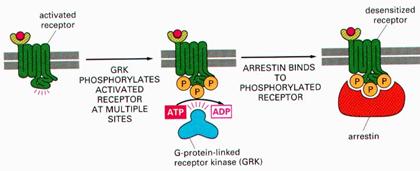
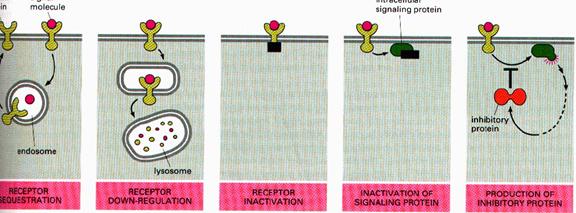
Down regulation of receptors:
Convergence:
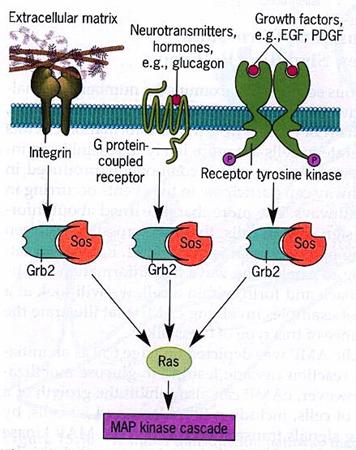
Divergence:
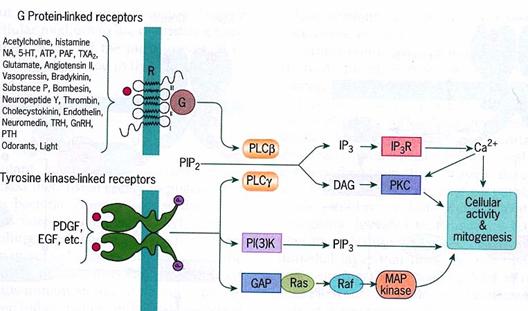
Cross-talk: