LYSOSOME
It is a membrane-bounded organelle, found in the cytoplasm of eukaryotic cells, which contains digestive enzymes. It acts as the "garbage disposal" of the cell by breaking down cell components that are no longer needed as well as molecules or even bacteria that are ingested by the cell. Lysosomes are organelles containing digestive enzymes (acid hydrolases). They are found in animal cells, while in plant cells the same roles are performed by the vacuole. The name lysosome derives from the Greek words lysis, which means dissolution or destruction, and soma, which means body. They are frequently nicknamed "suicide-bags" or "suicide-sacs" by cell biologists due to their role in autolysis. Lysosomes were discovered by the Belgian cytologist Christian de Duve in 1955.

Occurrence:
Lysosomes occur in most animal cells and in a few plant cells as vacuoles. They are most abundant in cells which are related with enzymatic reactions such as liver cells, pancreatic cells, kidney cells, spleen cells, leucocytes, macrophages etc.,
Shape:
Lysosomes are usually spherical in shape; but they are irregular in certain meristematic cells of roots.
Size:
The size of the lysosomes usually ranges from 0.2micron to 0.8micron in diameter, but it might present as large as 8 microns in mammalian kidney cells and macrophages.
Structure:
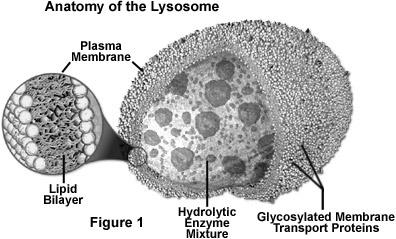
Lysosomes are single membrane bound organelle. They are round dense bodies filled with large number of hydrolytic enzymes and lytic components. Lysosomes are so called because they contain lytic enzymes. The enzymes are kept inside by the lysosomal membrane. These enzymes remain inactive inside the lysosomes. When the pH of the interior lysosomes changed to acidic pH 4.8, enzymes become active. At pH 4.8, the interior of the lysosomes is acidic compared to the slightly alkaline cytosol (pH 7.2). The lysosome maintains this pH differential by pumping protons (H+ ions) from the cytosol across the membrane via proton pumps and chloride ion channels. The lysosomal membrane protects the cytosol, and therefore the rest of the cell, from the degradative enzymes within the lysosome. The cell is additionally protected from any lysosomal acid hydrolases that leak into the cytosol as these enzymes are pH-sensitive and function less well in the alkaline environment of the cytosol.
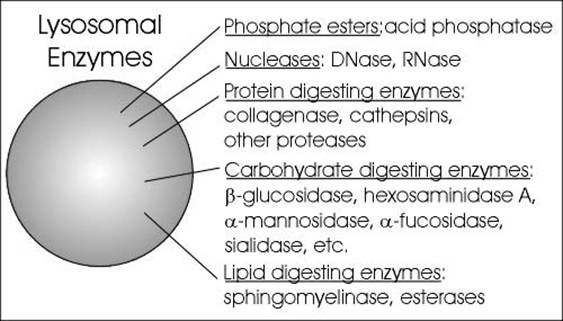
The lysosomal enzymes are collectively called as hydrolases. The hydrolases bring about the cleavage of substrates by the addition of a water molecules. Most of the lysosomal enzymes function in the acid medium. Hence they are called as acid hydrolases. The lysosomes may contain about 40 varieties of enzymes. The lysosomal enzymes are classified into six main types namely Nucleases, Proteases, glycosidases, lipases, phosphatases and sulphatases.
1. Nulceases:
Nucleases hydrolyse nucleic acids into nitrogen bases, phosphates and sugars. The nucleases are of two types namely deoxyribonuclease and ribonuclease. Deoxyribonuclease acts on DNA whereas ribonuclease acts on RNA.
2. Proteases:
Proteases hydrolyse proteins into amino acid residues. Example for proteases include cathepsin, collagenase and peptidases.
3. Glycosidases:
Glycosidases hydrolyse glycosidic bonds of polysaccharides into monosaccharides. Example: beta galactosidase which breaks down lactose into glucose and galactose.
4. Lipases:
Lipases hydrolyse lipids into fatty acids and alcohol. They include esterases and phospholipiases.
5. Phosphatases:
Phosphatases release phosphates from organic compounds with phosphates. These include acid phosphatases and acid phosphodiesterases.
6. Sulphatases:
Sulphatases release sulphates from the organic compounds with sulphate.
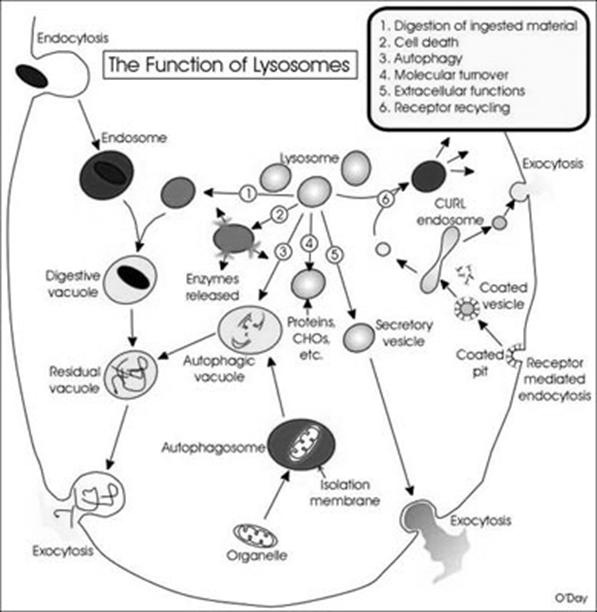
Lytic component present in lysosome include lactoferrin which act as iron chelators i.e. it removes iron from the lysoendosomal vesicle during the lytic function of lysosome. Lysosomes might be differentiated into four types depending upon their content during their function namely, primarly lysosome, secondary lysosome, residual body and autophagic vacuoles.
Primary lysosome are small saclike structures enclosing enzymes synthesized by the ribosomes. Since they store enzymes, they are also said to be storage granules.
Secondary lysosome are formed by the fusion of primary lysosome with phagosomes. They contain engulfed materials and enzymes. The materials are progressively digested by the enzymes. So it is also otherwise called as digestive vacuole.
Residual bodies are nothing but secondary lysosomes with undigested wastes. The digested materials are diffused into the cell cytoplasm through the lysosomal membrane.
Autophagic lysosomes are special type of lysosmes, which are formed when the cells feed on their own intracellular organelles and they digest them ultimately. This happens only during starvation of organisms.
Function:
Lysosomes have the following functions namely, Heterophagy, Autophagy, Autolysis, Extracellular digestion, Fertilization and chromosomal damage.
1. Heterophagy:
Heterophagy is the lysosomal digestion of extracellular materials by the process of endocytosis. Heterophagy are of two types namely phagocytosis and pinocytosis.
i) Phagocytosis:
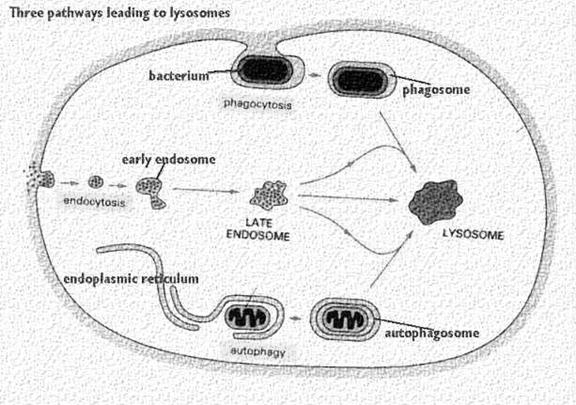
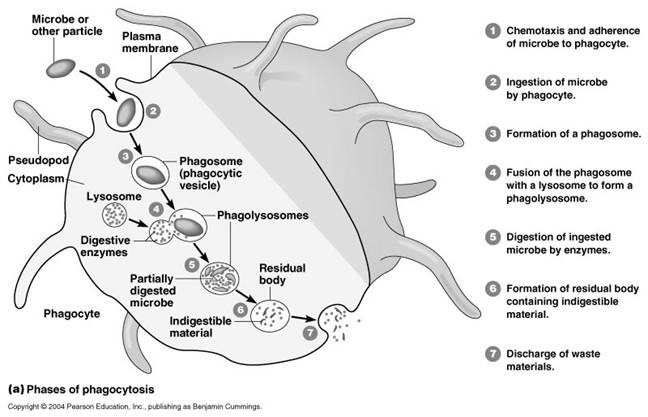
It occurs in five steps namely uptake, endosome formation, phagolysosome formation, lysis, diffusion of digested material and exocytosis. In order to uptake microorganism, pseudopodia developed arrount the microbe and takes up the microbe to form endosome.
Endosome again combines with lysosomal fraction to form phagoendosome. When the pH of the phagoendosome changed to acidic, lysosomal enzyme activated and they lyse microbes. After lysis, digested materials are passed through diffusion into cellular compoenent. Undigested materials during phagocytosis, will be excreted out.
i) Phagocytosis:
It occurs in five steps namely uptake, endosome formation, phagolysosome formation, lysis, diffusion of digested material and exocytosis. In order to uptake microorganism, pseudopodia developed arrount the microbe and takes up the microbe to form endosome.
Endosome again combines with lysosomal fraction to form phagoendosome. When the pH of the phagoendosome changed to acidic, lysosomal enzyme activated and they lyse microbes. After lysis, digested materials are passed through diffusion into cellular compoenent. Undigested materials during phagocytosis, will be excreted out.
Phagocytosis is one type of endocytosis, the general term for the uptake by a cell of material from its environment. In phagocytosis, a cell’s plasma membrane expands around the particulate material, which may include whole pathogenic microorganisms, to form large vesicles called phagosomes. Most phagocytosis is conducted by specialized cells, such as blood monocytes, neutrophils, and tissue macrophages. Most cell types are capable of other forms of endocytosis, such as receptor-mediated endocytosis, in which extracellular molecules are internalized after binding by specific cellular receptors, and pinocytosis, the process by which cells take up fluid from the surrounding medium along with any molecules contained in it.
The process of phagocytosis was discovered by Metchnikoff. The term phagocytic denotes the engulfment and digestion of whole cells. The two major cell types in the body which are associated with the engulfment and digestion of microorganism are the polymorphonuclear leucocytes and the macrophages. Minor cell types are eosinophils. The process of phagocytosis involves the following steps:
a. Attachment:
Attachment is the adherence of bacterium to the cell membrane of the phagocytic cell. Some bacteria are easily attached to the phagocytic cell example mycobacterium tubercle. Some other requires that they be coated with antibody before attachment. This is called opsonization. Thus, antibody functions as an opsonin, a molecule that binds to both antigen and macrophage and enhances phagocytosis.
b. Phagosome formation:
After attachment the phagocyte extent small pseudopodia around the infecting bacterium, the pseudopodia fuse to form a pouch which contain a bacterium surrounded by the cell membrane. This structure is called as the phagosome.
c. Phagolysosome formation:
After engulfment in the form of phagosome, the lysosome containing the hydrolytic enzymes fuses with phagosome to form phagolysosome. In this step, lysosomal enzymes discharged to phagosome which is vital for the lysis of bacteria.
d. Lysis:
A number of antimicrobial and cytotoxic substances produced by activated macrophages can destroy phagocytosed microorganisms.
OXYGEN-DEPENDENT KILLING MECHANISMS:
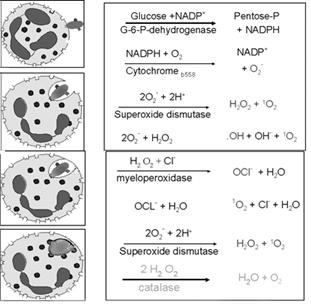
Activated phagocytes produce a number of reactive oxygen intermediates (ROIs) and reactive nitrogen intermediates that have potent antimicrobial activity. During phagocytosis, a metabolic process known as the respiratory burst occurs in activated macrophages. This process results in the activation of a membrane-bound oxidase that catalyzes the reduction of oxygen to superoxide anion, reactive oxygen intermediate that is extremely toxic to ingested microorganisms. The superoxide anion also generates other powerful oxidizing agents, including hydroxyl radicals and hydrogen peroxide. As the lysosome fuses with the phagosome, the activity of myeloperoxidase produces hypochlorite from hydrogen peroxide and chloride ions. Hypochlorite, the active agent of household bleach, is toxic to ingested microbes.
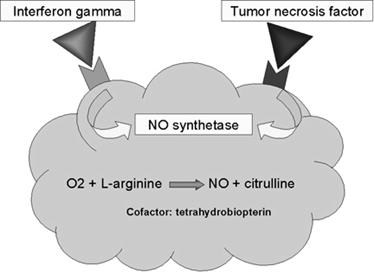
When macrophages are activated with bacterial cell-wall components such as lipopolysaccharide (LPS) or, in the case of mycobacteria, muramyl dipeptide (MDP), together with a T-cell–derived cytokine (IFN-g), they begin to express high levels of nitric oxide synthetase (NOS), an enzyme that oxidizes L-arginine to yield L-citrulline and nitric oxide (NO), a gas:
L-arginine + O2 +NADPH/H+ → NO + L-citrulline + NADP+
Nitric oxide has potent antimicrobial activity; it also can combine with the superoxide anion to yield even more potent antimicrobial substances. Recent evidence suggests that much of the antimicrobial activity of macrophages against bacteria, fungi, parasitic worms, and protozoa is due to nitric oxide and substances derived from it.
OXYGEN-INDEPENDENT KILLING MECHANISMS:
Activated macrophages also synthesize lysozyme and various hydrolytic enzymes whose degradative activities do not require oxygen. In addition, activated macrophages produce a group of antimicrobial and cytotoxic peptides, commonly known as defensins. Cathepsin G is an example for defensins. These molecules are cysteine-rich cationic peptides containing 29–35 amino-acid residues. Each peptide, which contains six invariant cysteines, forms a circular molecule that is stabilized by intramolecular disulfide bonds. These circularized defensin peptides have been shown to form ion-permeable channels in bacterial cell membranes. Defensins can kill a variety of bacteria, including Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Haemophilus influenzae. Activated macrophages also secrete tumor necrosis factor (TNF), a cytokine that has a variety of effects and is cytotoxic for some tumor cells. Lactoferin chelates iron from the medium and prevent the growth and proliferation of iron dependent bacteria. Lysozyme splits mucopeptide in bacterial cell wall and lysed bacteria.
e. Exocytosis:
Finally the killed organisms are digested by hydrolytic enzymes and the degraded products are released to the exterior by the process of exocytosis.
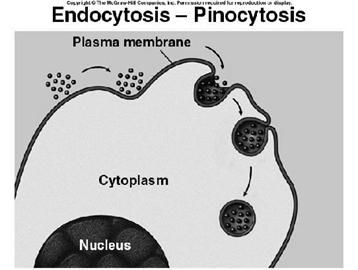
ii) Pinocytosis:
pinocytosis is the process of engulfment of fluid particles through the plasma membrane. It was first identified by Lewis. During pinocytosis, the plasma membrane is invaginated to form sac-like structures. The fluid material is drawn into the sac. Then the sac is pinched off from the plasma membrane forming a vesicle called pinosome. The pinosome later fuses with lysosomal vesicle. Then the material inside the pinolysosome was lysed by the action of lysosomal enzymes. The digested material diffuses into the cytoplasm
2. Autophagy:
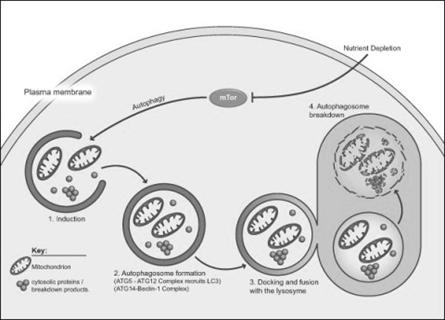
Autophagy is essential in helping to maintain the balance between the increase and decrease in the number of a cell population. It is undoubtedly active at a basal level in most cells and contributes to the routine turnover of cytoplasmic components. Three predominant cellular functions can be assigned to autophagy. Autophagy is a response to nutrient starvation. Decreased levels of amino acids can induce the autophagic response in numerous cell types and situations e.g. the neonatal period, when the supply of nutrients via the milk has not yet replaced the nutrients via the placenta.
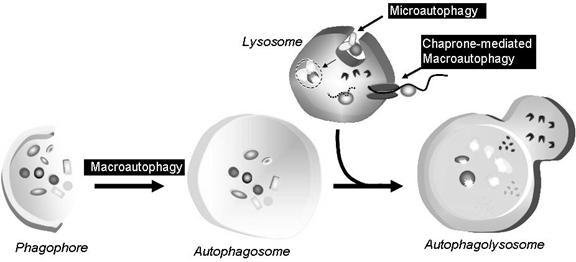
Autophagy is a housekeeping process whereby long-lived proteins and organelles are recycled e.g. Mitochondria. Autophagy has tissue-specific roles e.g. during erythrocyte development, following nucleus expulsion, autophagy is required to degrade the remaining organelles. Degradation of the autophagic vesicle results in the functional biconcave shape. The term ‘Autophagy’ covers three processes; microautophagy, macroautophagy and chaperone-mediated autophagy:
Microautophagy is the transfer of cytosolic components into the lysosome by direct invagination of the lysosomal membrane and subsequent budding of vesicles into the lysosomal lumen.
Macroautophagy involves formation of a double-membrane structure called the autophagosome which sequesters cytosolic material and delivers it to the lysosome for degradation. Although this degradation can be selective (i.e. specific removal of damaged mitochondria sparing normal functioning ones), degradation of soluble cytosolic proteins is non-selective.
Chaperone-mediated autophagy (CMA) is characterised by its selectivity regarding the specific substrates (cytosolic proteins) degraded.
3. Autolysis:
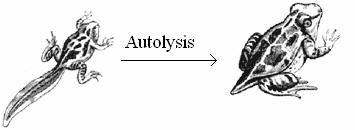
Autolysis refers to the digestion of own cells by the lysosomes. Auto means self and lysis means digestion. It is self digestion. It is also otherwise known as programmed cell death or apoptopic lysis. In autolysis, the lysosome digests its own cell. Hence autolysis is also called as cellular autophagy. In this process, the lysosome ruptures inside its cell and the released enzymes digest and degrade the cell. As lysosome kills its own cell, it is called as sucidal bag.
Autolysis occurs during amphibian metamorphosis, insect metamorphosis, mensuration etc., During amphibiam metamorphosis, the cells in tail, gills etc., are digested by autolysis. Similarly during menstruation the cells in the uterine epithelium are lysed by autolysis.
4. Extracellular digestion:
Digestion of materials outside the cell is called extracellular digestion. In certain occasions lysosomes release enzymes outside the cell by exocytosis and bring out digestion. Extracellular digestion takes place during bone erosion process. Osteoclast cell of bone contain more number of lysosomes. These cells when release their lysosomal content on the surface of the bone, lysosomal enzymes bring about the extracellular digestion of bone and it result in bone desorption.
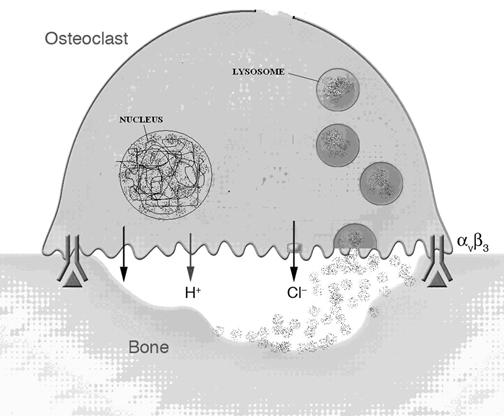
5. Fertilization:
During fertilization process, acrosome (giant lysosome) of sperm head ruptures and releases enzymes on the surface of the egg. These enzymes digest the egg membrane and provide way for the entry of sperm nucleus into the egg. This action also activates the egg for the developmental processes.
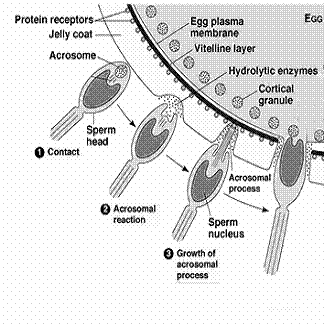
6. Chromosomal damage:
Due to the presence of DNase enzyme, lysosome had an ability to attacks chromosome and cause chromosomal breakages. These breakages can leads to diseases like cancer etc., .