THE MAP KINASE PATHWAY
Several receptors i.e., especially those involved in growth control, cell differentiation and the inflammatory responses, have either a) tyrosine kinase activity or b) are associated with proteins that are tyrosine kinases. An important feature of these hormones is that, these kinases preferentially phosphorylate Tyr (Y) residues. Otherwise, Tyr phosphorylation is infrequent (< 0.03%) in mammalian cells, when compared to serine and threonine phosphorylation.
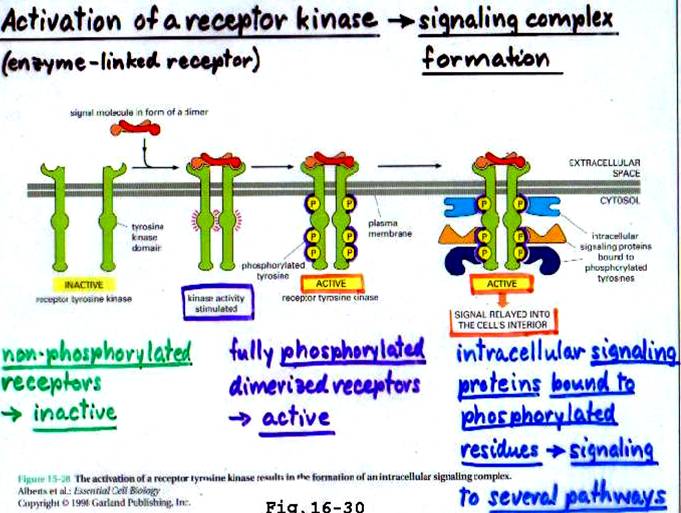
Some hormone receptors like those of insulin, endothelial growth factor (EGF) and Insulin – like growth factor (IGF – 1), have intrinsic tyrosine kinase activity. Activation of this kinase results in the phosphorylation of protein substrates on tyrosine residues. This results in a cascade (step) of events that alter the biochemistry and/or molecular biology of the target cell. (see diagram pg 2)
6 classes of enzyme-linked receptors have thus far been identified: -
a) Receptor tyrosine kinase (RTK)
b) Tyrosine-kinase-associated receptor
c) Receptor-like tyrosine phosphatases
d) Receptor serine/threonine kinase
e) Receptor guanylyl cyclases
f) Histidine-kinase-associated receptor
Of these, the signaling ligands of RTKs are as follows: -
a) Nerve growth factor (NGF)
b) Platelet-derived growth factor (PDGF)
c) Fibroblast growth factor (FGF)
d) Epidermal growth factor (EGF)
e) Insulin and insulin-like GF (IGF-1)
f) Ephrins (Eph)
g) Vascular endothelial factor (VEGF)
1. RECEPTOR TYROSINE KINASES (RTK)
Receptors with intracellular kinase activity can be divided into 2 groups by the substrate specificity of their kinase domains. Most of these receptor kinases phosphorylate other proteins on tyrosines, but some receptor kinases phosphorylate serines and threonines, such as the transforming growth factor receptors (TGFR).
2. RTK – CLASSIFICATION
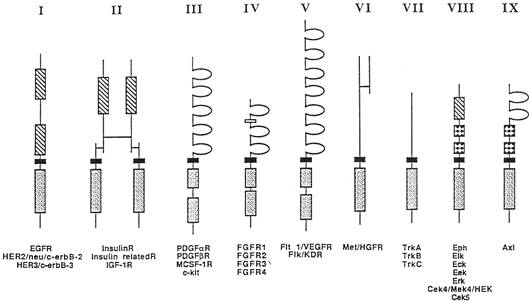
a. The receptor tyrosine kinase family can be further subdivided in various ways. One classification based on structural features groups the receptor tyrosine kinases into 9 subfamilies (see diagram pg 3).
b. There are also receptor tyrosine kinases which do not fit into any of these subfamilies.
c. Most of the receptor tyrosine kinases are single chain polypeptides containing only one transmembrane domain. However, there are a few exceptions, such as the insulin receptor family which consists of disulfide bonded heterotetramers and the hepatocyte growth factor receptor (HGFR) family where the molecules contain large extracellular domains that undergo proteolytic clevage near the N-terminus.
d. The small N-terminal fragment remains disulfide bonded to the major part of the receptor.
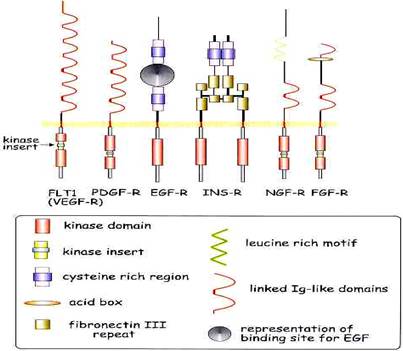
3. RTK – COMMON STRUCTURAL FEATURES

The following structural features are identified: - Tyrosine kinase domains (stippled boxes), transmembrane domains (solid boxes) cysteine-rich domains (striped boxes), immunoglobulin-like domains (semicircles), acidic box domain (open box), and fibronectin type 3 domains (checkered boxes).
4. RTK – TYPES OF ACTIVATION
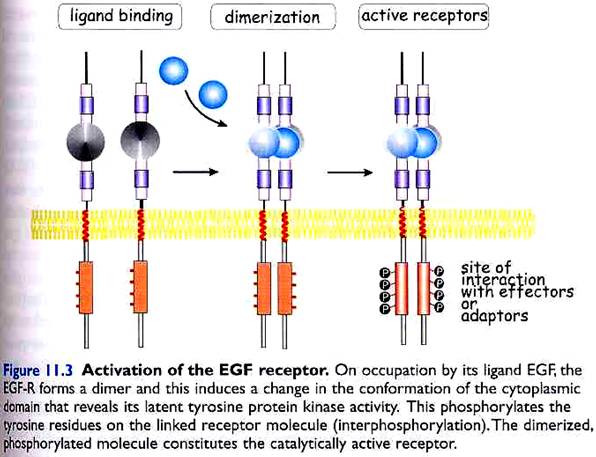
Receptor tyrosine kinases are thought to be activated by receptor dimerization or oligomerization. There are several different mechanisms by which ligand binding can induce receptor dimerization.
I. Several of the ligands are dimeric molecules, such as platelet derived growth factor (PDGF), colony stimulating factor 1 (CSF-1) and stem cell factor (SCF). Each of the monomeres of the dimeric ligand contains one receptor binding epitope, so that one dimer can bind two receptor molecules and thereby dimerize the receptor.
II. Other ligands, like epidermal growth factor (EGF) exist as monomeres, but can nevertheless bind simultaneously to two receptor molecules, and thereby induce dimerization. (see diagram below)
III. A third mechanism by which ligand binding can induce dimerization is the one utilized by FGFs. Several ligand molecules bind to one glycosaminoglycan molecule and each of the ligand molecules simultaneously bind to one receptor molecule, thereby dimerizing or oligomerizing the receptors.
IV. A fourth mechanism of receptor dimerization is used by the Eph related kinases. In this case, the ligands are normally membrane bound molecules. In soluble form the ligands do not activate the receptors, while antibody mediated receptor clustering does. Membrane attachment of the ligand appears to facilitate receptor dimerization.
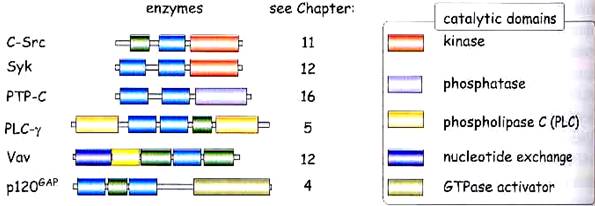
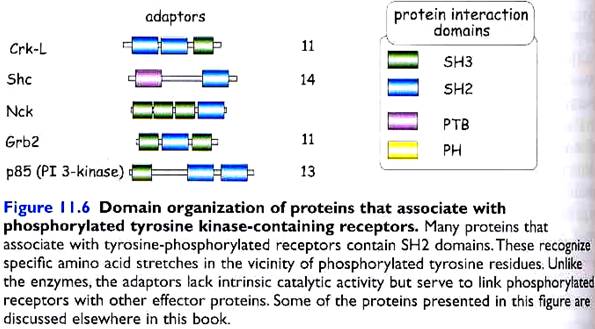
5. ADAPTER PROTEINS
Adapter proteins do not contain catalytic domains, but they serve to link phosphorylated tyrosine receptors to other effector proteins. E.g. Shc (SH2 domain and collagen-like), FRS2 (FGF receptor substrate 2), Grb2 (growth factor receptor bound 2), p85 (PI 3-kinase), mSOS (mammalian son of sevenless), etc. (see diagram pg 6)
When two (or more) receptor tyrosine kinase molecules are brought together in close physical contact, they can phosphorylate each other (so called transphosphorylation), possibly due to a low basal kinase activity.
Often the first tyrosine to be phosphorylated is a conserved tyrosine within the kinase domain itself. Phosphorylation of this tyrosine increases the kinase activity of the receptor and then several other tyrosines (often outside the kinase domain) are phosphorylated.
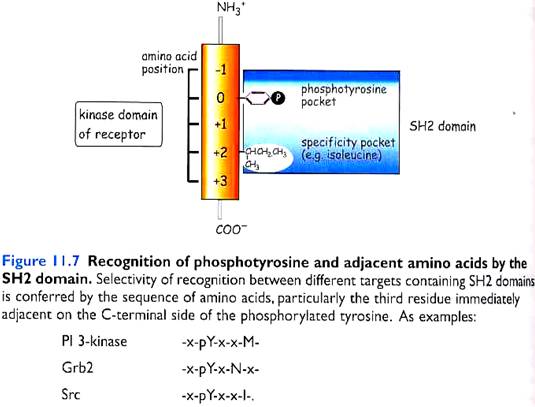
These phosphorylated tyrosines act as docking sites for Src-Homology 2 (SH2) domain containing proteins or PhosphoTyrosine Binding (PTB) domain containing proteins. These 2 are called protein interaction domains. SH3 and PH are other known domains.
SH2-domains are peptide segments of about 100 amino acids that are capable of binding to phosphorylated tyrosines. The specificity of binding is determined by the three amino acids (+1, +2 and +3) immediately in the C-terminal to the phosphorylated tyrosine. Several signal transducing molecules contain SH2-domains.
PTB domains consist of 100-150 amino acids and are found in signaling molecules like insulin receptor substrates 1 and 2 (IRS-1 and IRS-2) and Shc.
C What are some of the active domains for binding and kinase/phosphatase activity?
6. MITOGEN – ACTIVATED PROTEIN KINASE (MAPK) and SIGNALING PATHWAYS
§ MAP kinases are actually a family of protein kinases that are widely distributed and are found in all eukaryotes.
§ The mitogen activated protein kinase pathways (MAPKs) represent highly conserved cascades activated in response to effectors downstream of receptor tyrosine kinases (RTKs) or in some cases G-protein coupled receptors (GPCRs), and in response to inflammatory cytokines and stress. Together, they control many cellular processes.
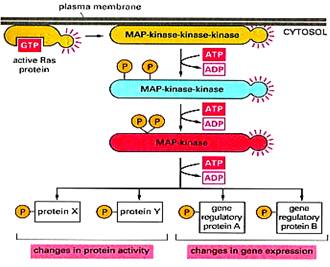
§ The hallmark of MAPK pathways is the shared architecture of a three-tier signaling core, namely, MAP3K/MAP2K/MAPK.
§ The downstream tier and main effectors MAPKs are activated by phosphorylation of threonine and tyrosine residues within a conserved TxY motif in the activation loop by dual specificity kinases MAP2Ks of the second tier, in turn activated by MAP3Ks serine/threonine kinases of the third tier.
§ The MAPKs are serine/threonine kinases whose targets include transcription factors and regulators, other nuclear or cytoplasmic proteins, kinases collectively known as MAPKAPK that further transmit the signal.
§ Docking interactions between members of the three tiers and with substrates and interactions with scaffold proteins assure the organization of distinct MAPK pathways and regulate the specificity of their signaling. Phosphatases and other regulatory proteins along with feedback loops act to shape these signaling cascades.
§ Over the last 20 years, 4 major pathways have been characterized, together comprising 20 MAP3K, 7 MAP2K and 11 MAPK.
§ Raf/Mek/Erk pathway, also known as the extracellular signal regulated MAPK is a well studied and characterized pathway acting downstream of RTK via small G protein Ras activation.
§ C-Jun N-terminal kinase (JNK) and p38 MAPKs pathways, known as the stress regulated MAPK cascades (SAPKs) are triggered by various stresses and are activated by adapters downstream of receptors for inflammatory cytokines. A subset of JNK can be triggered via small G protein Rho activation downstream of RTK or of G-protein coupled receptors (GPCR).
§ Finally, the Erk5 pathway (Extracellular signal regulated protein kinase) also known as the big MAP kinase is activated by various stresses but not by inflammatory cytokines and also not by growth factors in HeLa and PC12 cells.
§ Each of these pathways leads to the dual phosphorylation of MAP kinase family members responsible for activation of transcription factors.
 ABBREVIATIONS
(for the previous paragraph)
ABBREVIATIONS
(for the previous paragraph)
Ø MAPK – mitogen activated protein kinase
Ø MAP2K – mitogen activated protein kinase kinase, MAPKK (MEK)
Ø MAP3K – mitogen activated protein kinase kinase kinase, MAPKKK
Ø MAPKAPK – MAPK-activated protein kinase
Ø GF – growth factors
Ø Ras – Ras family of small G protein Ras superfamily
Ø Rho – Rho family of small G protein Ras superfamily
Ø Traf – TNF associated factor protein family
Ø RTK – receptor tyrosine kinase
Ø GPCR – G-protein coupled receptors
(Fig: General mechanism of MAPK pathway)
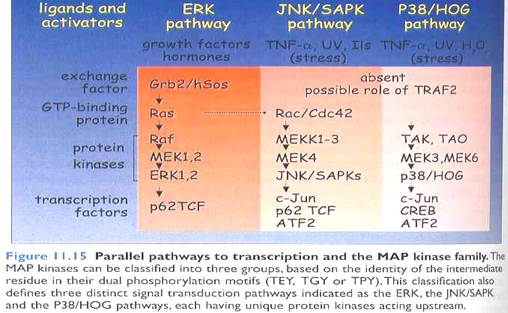
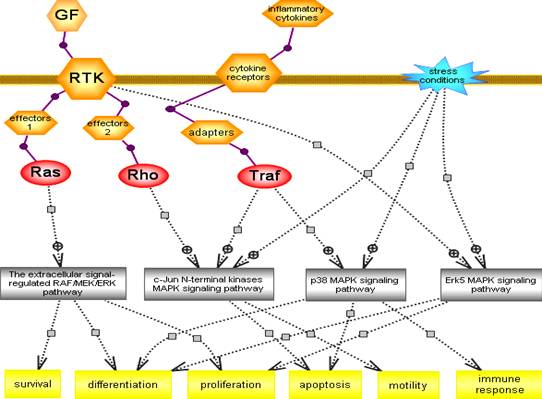
(Fig: Simple representation of various MAPK signaling)
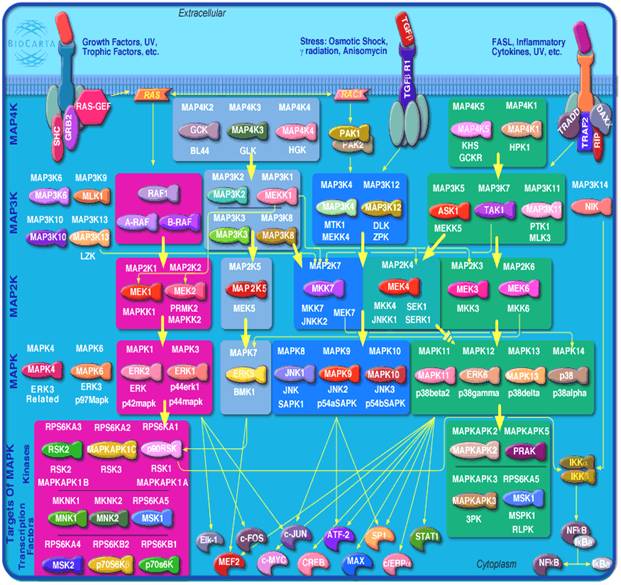
(Fig: MAPK pathways)