MINERALS
Introduction:
The importance of minerals in our well-being is emphasized by the fact that iron deficiency anemia is one of the three major health problems in India. The increase in the number of fractures in the elderly as also the incidence of high blood pressure are also pointers that focus one’s attention on minerals, calcium and sodium. A liberal intake of calcium, with increased mobility and exposure to sunlight is known to improve the strength of bones and thus reduce their fragility. A reduction in sodium intake can be one of the helpful factors in reducing hypertension. Though advertisements for dietary supplements may lead you to believe that these may be the answer to the problem it is not so. The key is to select foods to meet the body’s mineral needs and to season foods moderately to avoid excess intake of salt.

Nature and composition:
Minerals are present in all body tissues and fluids. In bones and teeth the minerals calcium and phosphorus are deposited in protein material. Iron is found in blood as a part of the red pigment, hemoglobin. Minerals occur in foods as salts and also in combination with organic substances.
Minerals have two distinct characteristics
o Mineral elements do not provide energy
o Mineral elements are not destroyed during food preparation
The mineral elements found in the body from only 4 to 6% of the weight of our adult body. This means that about 2 to 3kg of our body weight consists of minerals. Of this 90% is accounted for by seven minerals (calcium, phosphorus, potassium, sulphur, sodium, chloride and magnesium). The other minerals are known as trace elements as these add together to about 10% of the total mineral content of the body. The largest concentration of minerals is found in the bones and teeth. Minerals are also found in soft tissues such as nerves and muscles and in blood and other body fluids.
General Functions of Minerals:
Some minerals play an important role in the regulation of body functions. These are:
Minerals do not act singly in their function and regulation of body processes, but work with the help of other minerals and organic compounds. A certain concentration of each element must be present for efficient functioning of the body. Some of the important tissue formations and processes in which mineral elements function in unison were discussed below. This will be followed by a discussion of each important mineral element and its specific functions.
Bones and Teeth Formation:
Most of the calcium, phosphorus and magnesium and small amounts of other mineral elements are deposited in the bones and teeth. Bones and teeth are formed of tough protein material into which minerals are deposited. Most of the bone formation (ossification) in the fetus occurs in the eighth and ninth months of pregnancy. At birth, the bones are very soft, but the infant has a well formed skeleton. Throughout the growth phases (childhood, adolescence and early adulthood) the bones become long, thick and increase in hardness.
Thus bones form an important part of the body framework. Bones also serve as a reservoir of the component mineral elements. Thus the blood levels of these minerals are maintained by withdrawal of these elements from the bone. The minerals provided in the diet replace those withdrawn and thus help to maintain the bone structure. Thus even in boens, there is a continuous process of maintenance and repair of the tissue.
In the foetus, the first teeth are formed from fourth to sixth week of pregnancy. By the twentieth week, these teeth calcify. These are milk teeth and their formation is known as primary dentition.
Soon after birth up to about third birthday, the permanent teeth are formed, while wisdom teeth are formed between the eighth and tenth year. Before the teeth erupt, they are fully formed. Tooth enamel and dentine contain an appreciable quantities of calcium and phosphorus. These are protective layers of the teeth. As these parts do not contain blood vessels, a decayed tooth cannot repair itself. Hence the only way to ensure the health of teeth is to take care of the teeth and prevent decay.
Structural components of soft tissues:
Many mineral elements are found in the structural components of soft tissues. These include potassium, sulphur, phosphorus, iron and others.
As components and co-factors of vitamins, Hormones and enzymes:
Various regulatory compounds contain very small amounts of mineral elements as constituents. For example, sulphur is part of many important compounds such as thiamin and coenzyme-A. Vitamin B12 contains cobalt; the enzyme carbonic anhydrase contains zinc and the hormone thyroxine contains iodine. Calcium, as an activator, is a co-factor in the action of pancreatic lipase. Incorporation of iron into haemoglobin needs copper, while the synthesis of insulin in the pancreas needs zinc.
Muscle contraction and Response of Nerves:
These are regulated by the mineral elements (sodium, potassium, calcium and magnesium), present in body fluids in definite amounts. These elements regulate the movement of materials through the cell membrane. Normal response of nerves to physiological stimuli is also regulated by these mineral elements.
Control of water balance:
Sodium and potassium are responsible for the control of water balance between the inside and outside of cells. This function is dependent on the correct concentrations of sodium and potassium. Potassium is mainly found in the fluid inside the cell, while sodium is chiefly found in the fluid outside the cell.
Maintenance of Acid-Base balance:
It is very important to maintain a constant pH in the body fluids at all times to ensure normal function of our body. The minerali elements are responsible for the maintenance of acid-base balance. The pH of the body fluids is maintained between a narrow range of 7.35 and 7.45.
Recommended Dietary Allowances (RDA):
The recommended dietary allowance (RDA) for each mineral element for the adult is given later. The allowances for growth stages of life are higher per unit body weight than the adult stage. Unlike the major nutrients (carbohydrates, fats and proteins) which have high bioavailability (about 90%), mineral elements vary a lot in the percentage that is absorbed from the diet. Thus iron absorption may be as low as 5%; calcium 20%, while sodium is absorbed almost completely.
Absorption of mineral elements is favored by
Absorption of mineral elements is reduced by:
Toxicity:
The possibility of toxicity due to excessive intake of mineral elements from normal natural foods is very remote.
Excessive intake, which can be toxic, is possible only when
Even common salt used in the kitchen can be toxic, if fed by mistake in excess to infants.
NUTRIENT METABOLISM – MARTIN KOHLMEIER (Academic Press)
Calcium:
Nutritional summary
Function: Calcium is the major mineral in bone. It is needed for intracellular and hormone-like signaling, neurotransmission, muscle contraction, for the regulation of cell growth and differentiation, blood clotting, and many other functions.
Requirements: Adults should consume at least 1000mg/day; adolescents as well as postmenopausal and lactating women need slightly more.
Sources: Rich sources are all dairy foods, fortified cereals and juices, and tofu set with calcium sulfate. Kale and chard are more modest sources. Most plant-based foods contain much smaller amounts. The proportion that is absorbed from all sources strongly depends on vitamin D status (which increases calcium absorption and retention) and on phosphate, sodium, and animal protein intakes (excess decreases calcium absorption and retention).
Deficiency: Inadequate calcium availability slows bone growth and mineralization in childhood and adolescence, and causes bone mineral loss in adults. Low mineral content of bone (osteoporosis) greatly increases the risk of fractures. People with habitually low calcium intake may also have a greater than average risk of colon cancer and elevated blood pressure.
Excessive intake: Intakes over 2500 mg/day increase the risk of renal stone formation in some people.
Dietary sources
Significant calcium sources include dairy products, soybean curd (tofu), a few green leafy vegetables and calcium-fortified foods and beverages. Milk and yoghurt provide about I mg/ml, hard cheeses about 7 mg/g. Firm tofu contains 2 mg/g, and as much as 7 mg/g, if set with calcium sulfate. Other sources are green leafy vegetables, such as Chinese mustard greens (2.5 microg/g), kale (0.7 mg/g), and chard (0.6mg/g). Spinach also contains significant amounts of calcium (1.4 mg/g), but the high oxalate content minimizes absorption. Women in the US get around 600mg calcium from foods per day, and men between 800 and 900 mg, most of this from dairy products. Older people consume even less. Many people use dietary supplements in addition to food sources. Nonetheless, combined intakes of many are well below what is considered to be adequate.
Function
Signaling'. Calcium is a ubiquitous second messenger that links receptor-mediated hormone action to intracellular events. Binding of some effectors to their plasma membrane receptors activates associated calcium channels. The calcium-binding mediator calmodulin responds to calcium influx by activating various proteins, such as phosphorylase kinase (EC2.7.1.38), 1D-myo-inositol-trisphosphate 3-kinase (EC2.7.1.127), calcium/calmodulin-dependent protein kinase (EC2.7.1.123, phosphorylates vimentin, synapsin, glycogen synthase, myosin light-chains, and tau protein), or myosin lightchain kinase (EC2.7.1.117, in smooth muscle). Another type of receptor uses inositol triphosphate to release calcium from intracellular stores and activate protein kinase C.
Muscle contraction: Striated and other muscle cells respond to nerve impulses with a contraction. A widespread intracellular compartment, the sarcoplasmic reticulum, contains large amounts of calcium bound to calsequestrin, whereas the cytoplasma of a relaxed cell contains very little. Transmission of an action potential from a neuron end plate to the muscle cell triggers a torrential release of calcium from the sarcoplasmic reticulum into the cytoplasma and contraction begins. The contraction ends, because calcium-transporting ATPase (EC3.6.3.8) pumps calcium from cytoplasma back into the sarcoplasmic reticulum. This calcium pump is particularly effective, since it moves two calcium ions with each hydrolyzed ATE Fast-twitch skeletal muscle fibers contain a different calcium pump (ATP2A I ) than slow-twitch skeletal muscle fibers and cardiac muscle fibers (ATP2A2).
Neurotransmitter release: Calcium has important roles in the release of neurotransmitters from nerve terminals, but considerable uncertainties still exist about many details of the various mechanisms. Voltage-gated calcium channels (N-type, P-type, Q-type) allow the rapid influx of extracellular calcium into neurons in response to depolarization and this triggers neurotransmitter release. Neurotransmitter release and neuron excitability is also regulated by calcium influx through Maxi-K potassium channels.
Protein cofactor: Several enzymes are dependent on the presence of adequate calcium concentrations for full activity. One mode, outlined above, is the binding of calcium to calmodulin or another calcium-binding protein, which then activates specific target proteins, in other instances calcium acts directly on the protein. Numerous types of phospholipase A2 (EC3.1.1.4) require calcium as a direct cofactor, as do caldesmon kinase (EC2.7.1.120), phosphatidylinositol deacylase (EC3.1.1.52), 1,4-1actonase (EC3.1.1.25), protein-glutamine gamma-glutamyltransferase (transglutaminase, EC2.3.2.13), and calpain (EC3.4.22.17). Polypeptide N-acetylgalactosaminyltransferase (EC2.4.1.41) requires both calcium and manganese. A large group of metalloproteinases requires both calcium and zinc for the hydrolysis of various collagens, elastins, and other connective tissue proteins. Among these are stromelysin 1 (matrix metalloproteinase 3, EC3.4.24.17) and 2 (matrix metalloproteinase 10, EC3.4.24.22), matrilysin (matrix metalloproteinase 7, EC3.4.24.23), gelatinase A (matrix metalloproteinase 2, EC3.4.24.24) and B (EC3.4.24.35), neutrophil collagenase (matrix metalloproteinase 8, EC3.4.24.34), and macrophage elastase (matrix metalloproteinase 12, EC3.4.24.65). Apyrase (EC3.6.1.5), an ATP and ADP hydrolysing ectoenzyme, requires both calcium and magnesium.
Blood clotting: A web of fibrin aggregates and platelets forms around injured blood vessel endothelia. Calcium stimulates both platelet aggregation and several steps of the blood-clotting cascade. The protein chains of four of the clotting factors (fibrinogen and factors Vll, IX, and X) contain gamma-carboxylated glutamyl residues that bind calcium with high affinity. Lowering of calcium concentration (e.g. by addition of EDTA or oxalate to blood samples) disrupts blood clotting.
Cellgrowth: Calcium is involved in the initiation of DNA synthesis, in chromosome sorting, regulation of cell division and differentiation. The stimulation of the calciumsensing receptor, for example, promotes differentiation of cells such as keratinocytes.
5keletalstructure: The ability of vertebrate animals to move rapidly and with precise control is very much dependent on a rigid skeleton. As in all other vertebrates, calcium is the predominant cation of bones. Inadequate bone mineralization increases the risk of fracture. Bone mineralization is promoted and controlled by several extracellular calcium-binding proteins, including osteonectin, osteocalcin, and matrix Gla protein (both vitamin K-dependent), osteopontin, and decorin. A clear understanding of the sequence of events leading to bone mineralization is still lacking, however.
Vestibular system: The inner ear contains a sensory organ that perceives balance and gives information on body position. The sensory hair cells in the vestibular system detect the minute displacement of a layer of calcium carbonate crystals (otoconia) embedded in a gelatinous layer (otolithic membrane).
Phosphorus:
Nutritional summary
Function: Phosphate is the constituent anion of bone, participates in energy metabolism and storage (as ATE GTE creatine phosphate, arginine phosphate etc.), and is an important buffer in most body compartments.
Requirements: Adults should consume at least 700 mg/day; pregnancy and lactation do not increase needs.
Sources: Phosphate content of foods correlates somewhat with protein content, but distinct differences exist. Milk and dairy products have the highest phosphate to protein ratio (near 30 mg/g). Chicken and fish are at the low end (6-7 mg/g). Processed cheese, many other processed foods as well as colas and some other sodas are also important sources. Much of the large amounts of phosphate in plant-derived foods is bound to inositol (phytate and related forms) and is not absorbed. Food tables give the misleading impression that legumes, oats, rye, and other grains are major phosphate sources, which they are not.
Deficiency: Phosphate inadequacy is usually due to low food consumption or starvation, and is not rare in old people. Accelerated bone mineral loss causes osteoporosis and increases fracture risk.
Excessive intake: Phosphate intakes that significantly exceed calcium intakes (on a milligram basis) induce parathyroid gland hyperplasia and parathyroid hormone (PTH) secretion, impair vitamin D activation, and accelerate bone mineral loss and fracture risk. Extremely high intakes may cause calcification of extraosseous (non-bone) tissues, including arteries, kidneys, muscles, and tendons.
Dietary sources
It is important to recognize that foods contain three very different types of phosphate compounds. The first two types, inorganic phosphate salts and most organophosphates including phospholipids, are readily absorbed and profoundly impact human metabolism. In stark contrast, inositol polyphosphates are hardly absorbed at all, share virtually none of the metabolic characteristics of phosphate salts or other organophosphates, and have their own distinct properties. Current food tables and other sources of food composition data routinely provide only a single value for total phosphate and become virtually meaningless in the case of grains, legumes, fruits, and vegetables. Many (but not all) of these foods provide relatively little bioavailable phosphate, currently available food composition information and practice guidelines (ADA, 1998) notwithstanding. Milk (1.0 mg/g) and dairy products are major phosphate sources. Particularly concentrated sources are hard cheeses such as cheddar (5.1 mg/g) and swiss-type cheese (6.1 mg/g). Smaller amounts are in soft cheeses like cottage cheese (1.3 mg/g) or cream cheese (1.0 mg/g). An important measure is the ratio of bioavailable phosphate to protein, which ranges from very high in milk (29.6), hard cheese (around 20), and eggs (14.3), to much lower in pork (11.4), beef(9.5), chicken (6.7), and fish (6.1). This ratio can be an important tool for people who need to minimize phosphate intakes while maintaining adequate protein nutrition. The phosphate content of plant-derived foods is much more difficult to assess, since much of it is bound to inositol as phytate (inositol hexaphosphate), inositol pentaphosphate and inositol tetraphosphate.
Americans continue to increase their consumption of phosphate-containing food additives and may have average intakes from this source alone that approach 500 mg/day by now. Total daily phosphate intakes of young men tend to be around 1500 mg, that of young women around 1000. Intakes vary greatly between individuals and tend to decline after young adulthood. The above-mentioned caveat about the possible lack of relevance of total phosphate intake estimates, especially for groups with presumably healthful diets (rich in whole grains, legumes, fruits, and vegetables), has to be emphasized, however.
Function
High-enegy, phosphate esters: Most transfers of chemical energy in the body involve phosphate ester bonds. This is particularly true for ATP as the main metabolic energy currency, but also applies to more specialized forms, including GTP, CTE creatine phosphate, and arginine phosphate. These and additional nucleotides are also the essential building blocks for the synthesis of DNA and RNA. Several nucleotides, such as cAME are also critical for intracellular signaling.
Organophosphates: A large spectrum of endogenously synthesized compounds, aside from the ones mentioned above, contain one or more phosphate groups as an integral part of their structure. Large amounts of various types of phospholipids are needed for the construction of cell membranes, myelin-sheathing of neurons, transport of lipids with lipoproteins, facilitation of intestinal lipid absorption, among other functions. The phosphate-containing cholesterol precursors should also be mentioned.
Activating phosphate esters: The metabolism of many nutrients proceeds via phosphate esters at some point to provide the necessary reaction energy. Examples include the phosphates of glucose, fructose, galactose, glycerol, etc. The list of vitamins that are active only as phosphate esters includes thiamin, riboflavin, pyridoxine, niacin, and pantothenate. Phosphorylation can also serve to prevent efflux of such nutrients from cells (trapping) and aid uptake by passive diffusion. A typical example of such trapping is the intestinal absorption of vitamin B6. Free vitamin B6 diffuses across the intestinal brush border membrane via as yet unknown carriers and is then immediately phosphorylated by pyridoxal kinase (EC2.7.1.35). Since the phosphorylated form cannot return and intracellular concentration of the free forms is very low, diffusion into the enterocytes continues as long as there are significant amounts in the intestinal lumen.
Protein phosphorylation: The activity of many proteins is regulated through phosphorylation and dephosphorylation. The effect of an added phosphate group depends on the particular protein. Phosphorylation inactivates glycogen synthase (EC2.4. I. l l ), for instance, and dephosphorylation reactivates the enzyme.
Buffering: Aqueous solutions at physiological pH (around 7.4) contain about four-fifths of the inorganic phosphate as hydrogen phosphate ion (HPO42-), and nearly one-fifth
as dihydrogen phosphate ion (H2PO4-). This means that the significant amount of inorganic phosphate in cells, extracellular fluid, and blood acts as an effective buffer and stabilizes pH.
Polyphosphates: Some types of osteoblasts contain significant amounts of polyphosphates. These polyphosphates can contain up to several thousand phosphates linked into a chain. The function of these structures is not well understood. Suggested roles include phosphate storage, inhibition of bone mineralization, divalent ion and basic amino acid chelation, apoptosis, pH regulation, and protection against osmotic stress. Incompletely characterized exopolyphosphatases, and in some cases pyrophosphatase (EC3.6.1.1) release phosphate ions from the polyphosphate chains.
Pottassium:
N u t r i t i o n a l summary
Function: Potassium is the main cationic osmolyte within cells. The element plays a major role in body electricity (maintenance of cellular polarity, neuronal signaling, heart impulse transmission, and muscle contraction), nutrient and metabolite transport, and enzyme activation.
Food sources: Fruits and vegetables tend to contain large amounts of potassium, whereas animal-derived foods and grains provide smaller amounts.
Requirements: Healthy adults should consume at least 1600mg potassium per day. Increased losses, often due to use of certain diuretics or laxatives, have to be balanced with higher intakes. Inadequate intakes lead to low plasma potassium concentration (hypokalemia) with an increased risk of heart arrhythmia, muscle weakness, paralysis, alkalosis (increased blood pH), and eventually death. Higher than minimal (but not excessive) intakes are likely to be beneficial by lowering the risk of hypertension.
Excessive intake: When potassium intakes exceed the amount that can be removed (about 18g/day), the rising plasma concentration (hyperkalemia) can cause muscle weakness, interference with electric conductance in the heart (arrhythmia), and eventually death due to cardiac arrest. The risk of hyperkalemia is particularly high in patients with renal failure.
Dietary sources
Both plant and animal-derived foods are among the best sources. Potassium-rich foods of plant origin include avocados (6.3 mg/g), spinach (raw 5.6g), bananas (4mg/g), oats (3.5 mg/g), and rye flour (3.4 mg/g). Relatively potassium-rich animal products are halibut (5.8microg), tuna (5.7mg/g), mackerel (4.1 mg/g), and salmon (3.8mg/g). Slightly less is available in meats, such as pork (3.2 mg/g), beef (3.0 mg/g), and chicken (2.4 microg/g). The potassium content is relatively low in wheat flour (1.5 mg/g), eggs (1.2 microg/g), and cheeses (around 1 mg/g), and very low in rice (0.4 mg/g). Orange juice (2.0 mg/g) and milk (1.5 mg/g) are important sources because of the large consumed volumes. Losses with leaching during preparation or cooking of foods can be significant due to the high solubility of potassium salts in water. Boiled and drained spinach, for example, contains 17% less potassium than the same amount of raw spinach. The difference between raw and cooked kale is nearly 50%. Median intakes in the United States have been estimated at 3.2 g per day
Function
Excitation: The ability of neurons, muscles, and specialized excitable tissues in the heart to depolarize relies on the change in electric charge of the cells in response to a given stimulus. These cells have a negative charge of about 70-90 mV at rest because anion concentration is slightly higher inside than outside. The polarization rapidly flips when gated potassium channels first release potassium from these cells, followed by influx of sodium ions. The change in polarity propagates along the length of these excitable cells and can move to another excitable cell by direct electric stimulation or indirectly via a chemical transmitter (e.g. acetylcholine). Aberrations of potassium status cause typical electrocardiographic (ECG) changes. Patients with hypokalemia show a characteristic additional wave (U-wave) just preceding and in opposite direction to the normal T wave. Hyperkalemia flattens the P wave, lengthens the QRS interval, and sharpens the T wave.
Enzyme activation: Monovalent ions are unspecific activators for many enzymes. Pyruvate kinase (EC2.7.1.40) and pyruvate carboxylase (EC6.4.1.1) have a slightly more specific potassium requirement. There is no indication that variation of potassium concentration within normal ranges alters enzyme activities to a biologically significant extent.
Radioactivity: The isotope 40K is a g-emitter with a half-life of 1.3 -.109 years. It has been estimated that about 90% of the body's exposure to radioactivity is due to the estimated
17 mg of radioactive potassium.
Sodium:
Nutritional summary
Function: Sodium is the main cationic osmolyte in blood and extracellular fluid, and mediates active transport of numerous nutrients and metabolites in intestines, kidney, and many other tissues.
Food sources: Meats, pickled foods, and salty snacks are the major sodium sources.
Requirements: Intakes have to match sodium losses. Under most situations a balance can be achieved with daily intakes of a few hundred milligrams. Increased sweating (high with exertion, fever, heat, and high humidity) and diuresis can increase sodium needs to several grams per day. Very low sodium intake, for instance with fasting, can cause dizziness and weakness due to hypotension.
Excessive intake: Higher than minimal sodium intakes may increase blood pressure, especially in genetically susceptible individuals and when other hypertensive factors (obesity) are present.
Dietary sources
Most sodium in food comes from animal foods, added salt (NaCI), and sodium salts, such as monosodium glutamate (MSG), in commercial products. Sodium content of meats tends to correlate inversely with fat content. Chicken (0.86mg/g) and fish (0.78 mg/g) tend be higher in sodium than beef(0.57 mg/g) and pork (0.47 mg/g). Fruits, vegetables, tubers, and grains by themselves have very low sodium content (typically less than 0.1 g/kg).
Function
Enzyme cofactor: Because it is the main osmolyte in blood and the extracellular space, sodium concentration directly impacts volume. All enzyme-catalyzed reactions have specific requirements for ionic strength, which can be satisfied by sodium in the extracellular milieu. Beyond that, some reactions are specifically dependent on sodium ions.
Cotransport: Much of the movement of nutrients and metabolites is achieved by using the power of a sodium gradient. The enterocytes of the small intestine may serve as an example. Sodium/potassium-exchanging ATPase (EC3.6.3.9) at the basolateral membrane keeps the intracellular sodium concentration low by pumping sodium from the
enterocyte into the pericapillary space from where it diffuses into the bloodstream. The high sodium concentration in the intestinal lumen (from pancreatic and enteric secretions) creates a steep inward gradient. The sodium/glucose cotransporter I (SGT 1, SLC5A 1 ), like many similar cotransporters, moves sodium, water and glucose along that sodium gradient into the cell. This transport builds up enough of a glucose concentration in the enterocyte that it carries this substrate via glucose transporter 2 (GLUT2, SLC2A2) right across the basolateral membrane into the pericapillary space and eventually into the capillary bloodstream.
Signa/ing: Voltage-gated sodium channels are activated by the early phase of membrane polarization and allow rapid influx of sodium into neurons and muscle cells. This flux provides the driving force for continued membrane polarization.
Chlorine:
Nutritional summary
Function: Chloride is the main anionic osmolyte in blood and extracellular fluid, and contributes to active transport of some nutrients and metabolites in intestines, kidney, and other tissues. Hydrochloric acid in stomach contributes to protein digestion and inactivation of ingested microorganisms. Immune cells use directed release of hypochlorous acid to combat pathogens in blood and tissues.
Food sources: Meats, pickled foods, and salty snacks are the major chloride sources.
Requirements: Intakes have to match chloride losses. Under most situations a balance can be achieved with daily intakes of a few hundred milligrams. Increased sweating (high with fever, heat, and high humidity) and diuresis can increase chloride needs to several grams per day. Very low chloride intake, for instance with fasting, can cause dizziness and weakness due to hypotension.
Excessive intake: Higher than minimal chloride intakes may increase blood pressure, especially in genetically susceptible individuals and when other hypertensive factors (such as obesity) are present.
Dietary sources
Chloride intake closely correlates with sodium intake, since most chloride comes from animal foods and added salt (NaCI). The chloride content of meat tends to correlate inversely with fat content. Plant-derived foods are uniformly low in chloride. Some salt substitutes, such as potassium chloride, can be minor sources. While the UK food composition tables contain data on chloride (UK Food Standards Agency, 2001), US food composition databases lack this information.
Function
Electrolyte balance: Chloride is the major anion in extracellular fluids and helps to maintain normal osmotic pressure.
Transport: Several transporters require chloride as a cotransported counterion or exchange chloride for another anion. The taurine transporter (TAUT, SLC6A6), and the betaine transporter (BGTI, SLC6AI2) are examples that use both chloride and sodium. Chloride currents in other transporters, such as the excitatory amino acid carrier 1 (SLCIAI) responsible for terminating the neuroexcitatory action of L-glutamate, do not directly drive transport, but non-stoichiometrically regulate activity.
Acid production: Large amounts of chloride are used for the production of gastric acid. The hydration of carbon dioxide by the isoforms I and II of carbonate dehydratase (carbonic anhydrase, EC4.2.1.1, zinc-dependent) generates a prolific source of protons, in parietal cells of the stomach. The hydrogen/potassium-exchanging ATPase (EC3.6.3.10, magnesium-dependent) pumps these protons across the secretory canalicular membrane in exchange for potassium ions. The parallel export of chloride ions via the chloride channel 2 (CIC2) completes gastric acid secretion. The anion exchanger AE2 (SLC4A2) at the basolateral membrane stabilizes intracellular pH by removing the deprotonated bicarbonate anion in exchange for a new supply of chloride from the pericapillary fluid. The basolateral sodium/potassium/chloride cotransporter (NKCC 1, SLC 12A2s) provides an additional pathway for chloride influx. Chloride is also needed for acid production elsewhere, such as in endosomes for the recovery of filtered proteins in kidney (endocytotic pathway) or at the osteoclast ruffled membrane for bone mobilization. Distinct chloride channels are associated with the proton ATPases in kidney (chloride channel 5, CCL5) and bone (chloride channel 7, CCL7).
Enzyme activation: Peptidyl-dipeptidase A (angiotensin l-converting enzyme, ACE, EC3.4.15.1 ) and a few other enzymes have a stringent chloride requirement. The activity of most other enzymes is strongly dependent on ionic strength, to which the chloride concentration contributes significantly in extracellular space.
Immune defense: Myeloperoxidase (ECI.I 1.1.7) is a lysosomal hemoprotein in neutrophils, myelocytes, macrophages, and monocytes that uses chloride and hydrogen peroxide to generate hypochlorous acid (HOCI). The highly reactive hypochlorous ion is released as part of an oxidative blast of these immune cells that is normally directed at pathogens.
Magnesium:
Nutritional summary
Function: Magnesium (Mg) is an essential cofactor for a large number of reactions, including all of those involving ATP and GTP, participates in muscle and nerve depolarization, stabilizes DNA and RNA, and is a component of the mineral in bone.
Food sources: Whole grains, nuts and seeds, spinach, legumes, potatoes, and bananas are good sources. Hard tap water and some bottled mineral waters also can be significant sources.
Requirements: Men should get at least 400 mg (420 mg over age 30) from food, women at least 310 mg (320 mg over age 30, and 360 mg when pregnant).
Deficiency: Signs include confusion, disorientation, personality changes, loss of appetite, depression, muscle contractions and cramps, tingling, numbness, hypertension, abnormal heart rhythms, coronary spasm, and seizures. Deficiency is often induced by diarrhea, malabsorption, or vomiting, overuse of laxatives or diuretics, medication (cyclosporin, amphotericin, gentamycin, cisplatin), alcohol abuse, diabetes or hyperparathyroidism.
Excessive intake: Intakes of more than 350 mg from supplements and other nonfood sources may cause diarrhea, nausea, appetite loss, muscle weakness, mental impairment, difficulty breathing, extremely low blood pressure, and irregular heartbeat. Risk of toxicity is greater with impaired kidney function.
Dietary sources
Whole grains, nuts (66 mg/15 g), seeds (75 mg/30 g), spinach (65 mg/halfcup), legumes (35-54 mg/half cup), potatoes (55 mg/medium-sized), and bananas (34 mg/mediumsized) are good sources of Mg. Mg-rich mineral water may contain 110 mg/l or more. Typical daily Mg intakes of American women are around 220 mg; in men they are around 300 mg (Food and Nutrition Board, Institute of Medicine, 1997).
Function
Nucleotide complexes: All reactions that utilize ATP and similar nucleotides work efficiently only when these nucleotides are complexes with Mg.
Enzyme cofactor: There are also some enzymes that do not involve nucleotides as cofactors, but require Mg nonetheless. Manganese or other divalent cations are alternative cofactors for some of these enzymes. Their potential to substitute in vivo is in doubt, however, because of their low concentrations. Only a few examples of such enzymes will be given here. Phosphopyruvate hydratase (enolase, EC4.2.1.11 ) is both stabilized by Mg and requires the metal for catalysis. Phosphoglucomutase (EC5.4.2.2) is another enzyme in carbohydrate (galactose) metabolism that depends on Mg. The Mg-containing selenophosphate synthase (EC2.7.9.3) activates selenium prior to incorporation into proteins as selenocysteine. Choline synthesis and metabolism also depends on several Mg-requiring enzymes, including phosphatidylcholine synthase (EC2.7.8.24) and choline monooxygenase (EC1.14.15.7).
Bone mineral: Mg is a normal constituent of bone, but is not a constituent of the major mineral hydroxyapatite. The exact role of Mg in bone remains unclear.
Balancing calcium: The competition of Mg with calcium for binding to transporters, signaling structures and enzymes may be of functional importance. It has been suggested that excessive calcium availability and activity with decreasing Mg concentration may be responsible for a predisposition for muscle cramps, increased blood pressure, and coronary vasospasms. Use of supplemental Mg in at-risk women to prevent eclampsia, possibly through a similar mechanism, may be less essential than believed earlier.
Iron
Nutritional summary
Function: Iron is essential as a cofactor of oxygen transport, respiration, amino acid, lipid, alcohol, vitamin A, and sulfite metabolism, and various other redox reactions.
Requirements: At least 8 mg iron are necessary to maintain adequate stores for people consuming a mixed diet, more for women between 19 and 50 ( 18 mg/d), vegetarians, and during pregnancy (27 mg/d) and lactation (9 mg/d).
Sources: All muscle foods are good iron sources. Phytate from whole grains and some vegetables interferes with iron absorption; dietary ascorbate promotes absorption.
Deficiency: Diminished stores cause loss of appetite, microcytic anemia, and impaired immune function. Deficiency slows growth and cognitive development of infants and children. This may be partially irreversible.
Excessive intake: Greatly expanded iron stores may damage liver, pancreas, and heart. Iron supplement intake decreases the absorption of concomitantly ingested thyroxine, tetracycline derivatives, penicillamine, methyldopa, levodopa, carbidopa, and ciprofloxacin.
Dietary sources
Foods contain heine proteins (myoglobin, hemoglobin, cytochromes and a few enzymes), ferrous iron (Fe 2+), and ferric iron (Fe 3 +). Ferrous and ferric iron, usually called non-heine iron, comprises about 60% of the iron in meats, and most of the iron in plant-derived foods. Iron-rich foods include all meats, such as beef (0.019microg/g), pork (0.009mg/g), chicken (0.012 mg/g), and fish (e.g. tuna 0.009 mg/g). Legumes also are relatively iron-rich foods (e.g. baked beans 0.003 mg/g). Grains and grain products have to be fortified in the United States with 0.0044 mg/g. While iron intakes are very commonly inadequate worldwide, typical intakes tend to be high in North America and Europe. Median daily iron consumption of American women is around 9mg; median consumption of men is about 12 mg.
Function
Oxygen transport: One of the longest-known functions of iron is its role as a constituent of the oxygen-binding proteins hemoglobin and myoglobin. The tetrameric hemoglobin carries oxygen from the lung to peripheral tissues. Increased carbon dioxide concentration and associated high pH in oxygen-requiring tissues induce a conformational change (Bohr Effect) that releases the bound oxygen from hemoglobin and promotes the binding of carbon dioxide instead. Upon return of hemoglobin via blood circulation to the lungs the carbon dioxide is exchanged for another load of oxygen. Myoglobin is the main oxygen acceptor in muscle. Cytochromes are small proteins that figure importantly in the intmcellular transfer of redox equivalents for a wide range of acceptors.
Oxidative phosphorylation: Both heme and sulfur-bound iron act in the mitochondrial synthesis of ATE The NADH dehydrogenase (EC1.6.99.3) component of the respiratory chain complex I contains iron. The cytochrome c oxidase (ECI.9.3.1) subunits of the respiratory chain complex IV use hemes to accept electrons from ferricytochrome c and use them to reduce oxygen to water.
Free radical metabolism: Free iron readily transitions between its di- and trivalent forms, depending on its environment and potential reaction partners. It is a particularly strong catalyst for the generation of various oxygen free radicals. A typical example is the Fenton reaction that generates hydroxyl radicals ('OH) from superoxide anions (02). Fe 2+ can convert hydrogen peroxide, which is an intermediate of the Fenton reaction, into hydroxyl radicals. Atherosclerosis, cancer, and other chronic diseases have been tentatively linked to excessive iron concentrations. The iron enzyme superoxide dismutase (ECI. 15.1.1) and the heme enzymes catalase (EC 1.1 I. 1.6) and peroxidase (EC 1. I 1.1.7) keep the concentrations of superoxide, hydrogen peroxide, and other highly reactive oxygen-containing compounds in check.
DNA synthesis: Ribonucleoside-diphosphate reductase (EC 1.17.4.1 ), which contains both iron and ATE catalyzes the first step of DNA replication.
Nutrient metabolism: Several isoforms of the iron-containing alcohol dehydrogenase (ECI. 1.1.1) catalyze the metabolism of ethanol and of various nutrient metabolites.
Aldehyde oxidase (ECI.2.3.1) is one of several enzymes for the second step of ethanol catabolism. Aconitate hydratase (aconitase, EC4.2.1.3) is both an important Krebs cycle enzyme and a cytosolic iron sensor as described above.
VitaminA metabolism: Beta-carotene 15,15'-dioxygenase (ECI.13.11.21) is an ironcontaining enzyme in small intestine and liver that generates retinal from beta-carotene and a few other carotenoids. Another iron enzyme, retinal dehydrogenase (EC 1.2.1.36), produces retinoic acid.
Amino acid metabolism: The iron enzymes phenylalanine hydroxylase (EC 1.14.16. I ) and tyrosine 3-monooxygenase (EC I. 14.16.2) catalyze the initial step ofphenylalanine breakdown and catecholamine and melanin synthesis. Tryptophan 5-monooxygenase (ECI. 14.16.4) catalyzes the first step of serotonin synthesis. Trimethyllysine dioxygenase
(ECI.14.11.8) is involved in carnitine synthesis. Gamma-butyrobetaine, 2-oxoglutarate dioxygenase (ECI.14.11.1) participates in choline metabolism. The heme enzymes tryptophan 2,3-dioxygenase (ECI. 13.11.11 ) and indoleamine-pyrrole 2,3-dioxygenase (ECI. 13.11.42) facilitate tryptophan metabolism and niacin synthesis. The latter enzyme is also important for serotonin and melatonin degradation. The final step of taurine synthesis uses iron-containing hypotaurine dehydrogenase (EC1.8.1.3).
Thyroid hormones: The heme enzyme iodide peroxidase (ECI.I 1.1.8) oxidizes iodide, iodinates specific tyrosines in thyroglobulin, and couples iodinated tyrosines to generate thyroxin and triiodothyronine.
Protein-modification: Specific proteins are post-translationally modilied by several ironcontaining dioxygenases, including procollagen-proline 3-dioxygenase (ECI. 14. I1.7), procollagen-lysine 5-dioxygenase (ECI. 14.11.4), procollagen-proline, 2-oxoglutarate-4 dioxygenase (EC1.14.11.2), and peptide-aspartate beta-dioxygenase (ECI. 14.11.16).
Fatty acid metabolism: Two iron enzymes can add double bonds to fatty acids. Stearoyl-
CoA desaturase (EC1.14.99.5) produces oleic acid. LinoleoyI-CoA desaturase (EC1.14.99.25) catalyzes the first step of arachidonic acid synthesis (precursor of prostanoids) from linoleic acid.
5ulfur metabolism: Sulfite derives directly from the diet and from the metabolism of sulfur amino acids. Sulfite oxidase (ECI.8.3.1), which contains both heme and the molybdenum cofactor, converts sulfite to sulfate.
Dementia: Patients afflicted with Alzheimer's disease were found to have onset of their symptoms five years earlier, if they had the HFE variant H63D. This seems to indicate an increased disease risk in people with excessive iron stores. Acute poisoning: Accidental ingestion of multiple adult iron supplements is the most common cause of poisoning in infants.
Molybdinum:
Nutritional summary
Function: Humans need minute amounts of molybdenum for the metabolism of sulfite, nucleic acids, aldehydes, and taurine. Molybdenum may also have a role in the function ofglucocorticoid hormones and immune defense.
Requirements: Recommended intakes for adults are 45 microg/day (Food and Nutrition Board, Institute of Medicine, 2001). There is no indication that higher intakes are beneficial.
Food sources: Grain products, beans, and leafy vegetables are the best sources, but contents vary depending on the molybdenum content of the soil. Typical intakes in the US have been estimated to range from 50 to 100 microg/day.
Deficiency: It is very unlikely that healthy people show the metabolic disorders seen in severe experimental deprivation, which include xanthine kidney stones and impaired conversion of toxic sulfite to harmless sulfate compounds.
Excessive intake: Daily doses in the milligram range can cause gout. A number of other adverse effects, including reproductive failure, have been observed in animals with daily intakes over 5 mg/kg body weight; none of these adverse effects has been observed in humans. The tolerable upper intake level has been set at 2 mg/d (Food and Nutrition Board, Institute of Medicine, 2001).
Dietary sources
Foods contain molybdenum mainly as molybdopterin and MoO4 z-. Good molybdenum sources include milk (50 microg/l) and dairy products, legumes, especially soybeans, cereal products and leafy vegetables. Estimates of mean daily intakes vary widely, from 100-500 microg , 76 microg for women, and 109 microg for men.
Function
Molybdenum cofactor: Owing to its role as an essential cofactor of sulfite oxidase, aldehyde oxidases, xanthine dehydrogenase, and hypotaurine dehydrogenase, deficiency of molybdenum cofactor induces hypouricemia and increases urinary excretion of sulfite, thiosulfate, S-sulfocysteine, taurine, hypoxanthine, and xanthine, and decreases excretion of sulfate and urate. The deficiency may arise from a lack of molybdenum or from disrupted molybdopterin cofactor synthesis. Inborn lack ofcofactor synthesis is usually lethal in early childhood. Sulfite oxidase (Eel.8.3.1) is located in the mitochondrial intermembrane space where it converts sulfite into sulfate and generates hydrogen peroxide in the process; the enzyme contains both molybdenum cofactor and heme as prosthetic groups. Sulfite comes directly from ingested food (especially dried fruit and wine) and from the catabolism of cysteine (and indirectly of methionine). The peroxisomal enzyme xanthine dehydrogenase (EC1.1.3.22), which contains molybdenum cofactor, iron-sulfur clusters, and FAD, converts xanthine into uric acid. The enzyme in the liver normally functions as an NAD-dependent dehydrogenase. In other tissues the enzymes can be converted by oxidation or specific proteolysis into an oxidase, which does not require NAD, but generates hydrogen peroxide. The oxidative conversion of the enzyme into the oxidase form is reversible in the presence of sulfhydryl agents. Genetic deficiency of the enzyme is associated with unusually high excretion of xanthine and (xanthinuria) and formation of kidney stones rich in xanthine. Aldehyde oxidase (EC 1.2.3.1 ) contains molybdenum cofactor, heme, multiple iron/ sulfur clusters, and FAD. The cytosolic enzyme is especially abundant in liver, where it metabolizes acetaldehyde and other aldehydes to their respective acids. It may also oxidize retinal to retinoic acid. Aldehyde oxidase may be as important as the mixed-function hydroxylases for the hydroxylation or oxidation of various purines, pyrimidines, pteridines and related xenobiotics; an example is the conversion of the antiherpes agent famciclovir, a 9-substituted guanine derivative, to the active compound penciclovir.
Hypotaurine dehydrogenase (EC1.8.1.3), which contains molybdenum cofactor and heine, and is NAD-dependent, catalyzes the final step of taurine synthesis from cysteine (and the reverse reaction needed for taurine catabolism). Two additional molybdo-flavoproteins, AOH 1 and AOH2 (aldehyde oxidase homolog I and 2), have been identified based on their similarity to aldehyde oxidase and xanthine. AOH 1 has phenanthridine and benzaldehyde-oxidizing activity and is expressed in liver and in spermatogonia. AOH2 also has phenanthridineoxidizing activity and is expressed in keratinized epithelial cells and basal cells of skin and hair follicles.
Hormone-like effects: Molybdate stabilizes the glucocorticoid receptor (comprising heatshock proteins hsp56, hsp70, and hsp90), and inhibits the dexamethason-induced transformation to the DNA-binding state; the region 595 614 of the rat glucocorticoid receptor is a molybdate-binding site.
Other effects: High molybdenum intake may increase urinary copper losses, possibly by complexing copper. Tetrathiomolybdate is a synthetic compound that has been used as a copper chelator for the experimental treatment of Wilson disease.
Zinc:
Nutritional summary
Function: Zinc is essential for the activation of numerous genes and as cofactor for many enzyme reactions.
Requirements: At least 8 mg/d zinc are necessary to maintain adequate stores for women consuming a mixed diet, and 11 mg for men (Food and Nutrition Board, Institute of Medicine, 2002). Vegetarians and pregnant or breastfeeding women need slightly more.
Sources: Oysters are exceptionally rich, other shellfish and meats also are good sources.
Phytate from whole grains and some vegetables interfere with zinc absorption.
Deficiency: Low intake is associated with loss of appetite, scaling skin lesions, and impaired immune function.
Excessive intake: Consumption of more than 40mg/d may deplete copper stores, impair immune function, and lower HDL levels.
Dietary sources
Foods contain elemental zinc, much of it bound to proteins or DNA. Meats (2-3 mg/lO0 g), and shellfish (cooked clams 2.7 mg/100 g) provide most, oysters are exceptionally rich (>70 mg per serving). Plant foods are much poorer sources.
Function
Zinc is a cofactor of several hundred enzymes and is needed for the replication and function of DNA. Inadequate supplies impair food digestion and absorption, synaptic signaling, gene expression, control of oxidant stress, growth and wound healing, immune function, taste and appetite, and many other functions. Less than adequate zinc availability appears to interfere with organ formation during early pregnancy. It is often difficult to establish a tight link between individual zinc-dependent structures and functional status, because zinc impacts so many concurrent and sometimes competing processes. Only a limited selection will be touched on in the following.
Intestinal digestion: Zinc is an essential cofactor of carbonic anhydrases, proteases,
phosphatases and other enzymes involved in food digestion and absorption. The role of carbonic anhydrases for gastric acid production is described below. The carboxypeptidases A I and A2 (EC3.4.2.1) are zinc-dependent digestive enzymes from the pancreas. Several peptidases of the intestinal brush border are zinc enzymes, including leucine aminopeptidase (LAP, EC3.4. I 1.1), membrane alanine aminopeptidase (aminopeptidase N, EC3.4.11.2), glutamyl aminopeptidase (aminopeptidase A, EC3.4.1 !.7), membrane dipeptidase (EC3.4.13.19), angiotensin 1-converting enzyme (EC3.4.15.1), neprilysin (EC3.4.24.1 I), and meprin A (EC3.4.24.18). Another zinccontaining brush border enzyme (pteroylpoly-gamma-glutamate carboxypeptidase, EC3.4.19.8) is needed to cleave offgamma-glutamyl residues from dietary folate prior to absorption. Alkaline phosphatase (EC3.1.3.1, requires both zinc and magnesium) at the intestinal brush border digests complex forms of thiamin, riboflavin, and pantothenate.
pH-Regulation: The conversion of carbon dioxide to its weak acid helps cells to adjust proton concentration with a readily available and easily removable reagent. This equilibrium reaction is catalyzed by the zinc enzyme carbonate anhydrase (EC4.2.1.1). The ten or more genetically distinct isoforms of this handy enzyme provide fine tuned kinetic properties and regulatory characteristics for a wide range of functions that include acidification, signaling, promoting cell proliferation, bone resorption, and respiration. A long known role of the enzymes I, I1, and 1V is to provide protons for hydrochloric acid production in the stomach. Gastrin, histamine, and acetylcholine activate these isoenzymes, while somatostatin and several acid-suppressing drugs inhibit them. Carbonic anhydrase VI, gustin, is a special form in salivary glands, that helps to maintain taste bud growth (possibly by acting on bud stem cells) and function. Adequate zinc intake appears to promote taste acuity.
Nutrient metabolism: Zinc-containing alcohol dehydrogenases (ADH, EC 1.1.1.1 ) in the stomach wall and liver oxidize ethanol. A particular ADH isoenzyme is needed for the conversion of the transport and storage form retinol into the retinal form used for vision. Optimal zinc status may lower the risk of macular degeneration (Age-Related Eye Disease Study Research Group). Several zinc-dependent folate hydrolases in lysosomes, membranes, and cytosol are essential for folate metabolism and transport in tissues.
DNA replication and transcription: Zinc fingers are DNA-binding domains of transcription factors in which the zinc ion is tetrahedrally coordinated to cysteine and/or histidine residues. These zinc-complexing structures are very ubiquitous features that give zinc a central role in expression of virtually any type of protein. Zinc is a cofactor of many enzymes participating in the synthesis of DNA and RNA during cell division and gene expression. Important zinc enzymes include DNA polymerase (EC2.7.7.7) and DNA-dependent RNA polymerase (EC2.7.7.6); many others are regulated through zinc-finger proteins.
RNA editing: Zinc is a cofactor of the apolipoprotein B RNA editing complex, which deaminates specific cytidine moieties in a few mRNA species, most prominently of apolipoprotein B, but also of tumor necrosis factor-a, c-myc and other centrally important regulators of growth and differentiation (Anant and Davidson, 2000). The same complex also extensively edits the translational repressor NAT 1, which is involved in postnatal heart development.
Immune function: The zinc-dependent peptide hormone thymulin comes from the thymus. Thymulin helps to maintain immune function by activating T-lymphocytes and enhancing the cytotoxicity of natural killer cells. Zinc may also act directly by promoting the proliferation of lymphocytes and decrease susceptibility to programmed cell death (apoptosis). Since zinc is an essential cofactor of many enzymes involved in proliferation of any rapidly dividing cell, it becomes very difficult to differentiate this from a more specific role as a direct effector. Whatever the proximate mechanism, there is no doubt that counts of both natural killer cells and TH i lymphocytes decline with zinc deficiency. Related to suboptimal zinc are also low production of interleukin- 2, tumor necrosis factor-a, and interferon-gamma, and possibly of 11-6.
Fuel metabolism: The interactions of zinc with players in carbohydrate metabolism are numerous and not yet fully understood. Only a few are to be mentioned here. Zinc chelates insulin during storage and thus plays a role in control of its secretion. Glucagon rapidly lowers the intracellular concentration of the free zinc ion. Zinc itself opposes the effect of cAMP on glycolysis.
Free radical metabolism: Zinc contributes importantly to the defense against oxidative stress. It does so partly as a cofactor of superoxide dismutases (SOD, EC 1.15.1.1 ) in cytoplasma (SOD l) and in the extracellular space (SOD3). Protection of sulfhydryl
groups and other direct effects of zinc on redox reactions have been demonstrated.
Other functions: A link of zinc to vascular function is established through its role as a cofactor of nitric oxide synthases (EC 1.14.13.39).
Selenium:
Nutritional summary
Function: Selenium is a cofactor for enzymes and proteins with vital importance in antioxidant defense, thyroid hormone and insulin function, regulation of cell growth, and maintenance of fertility.
Requirements: Daily intakes of 55 microg are recommended for adults. Pregnant women should consume an additional 5 microg/d, lactating women an additional 4 microg/d (Food and Nutrition Board, Institute of Medicine, 2000).
Food sources: Seafood and liver are the foods with the highest selenium (Se) content; meat and grains contain less, and fruits and vegetables very little.
Deficiency: Deficiency increases the risk of cardiac failure, liver disease, cancer, atherosclerosis, cardiomyopathy (Keshan disease), and may cause hair loss, skin changes, and infertility.
Excessive intake: Continued consumption of 400 microg/d or more, especially with inorganic supplements, may cause peripheral neuropathy, nausea, diarrhea, dermatitis, hair loss, and nail deformities.
Dietary and other sources
Foods contain selenomethionine, selenocysteine, Se-methyl-selenomethionine, Semethyl-
selenocysteine, selenate, selenite; selenomethionine is the main form in plants, selenocysteine is the main form in foods of animal origin. The concentration of Se in foods is greatly dependent on the selenium content of the soil in which plants are grown and the Se content of animal feeds. Seafood and liver are the most Se-rich foods (400-1500microg/kg), meat (100-400 microg/kg), grains, vegetables, and fruits usually contain much less. Selenium intakes vary greatly depending on region and dietary habits, with typical US consumption between 70 and 100 microg per day. Selenides, selenates and various other inorganic Se compounds have significant toxic potential (at chronic intake levels of 1000 microg or more), mainly by causing dermatitis, hair loss, nail deformities, nausea, diarrhea, and peripheral neuropathy through unknown mechanisms.
Function
Antioxidant protection: Several enzymes that contain selenocysteine as a prosthetic group are involved in the maintenance of sulfhydryl groups in reduced form and the defense against oxygen free radical. Glutathione peroxidase (ECI. 11.1.9) designates four enzymes, which use reduced glutathione (GSH) for the reduction of peroxides of free fatty acids and other lipids. Glutathione peroxidase activity is crucial for the protection of membranes, proteins, and DNA against damage by lipid peroxidation and the fact that isoenzymes are encoded by at several distinct genes attests to its vital importance. The classical isoenzyme (GPXI) is ubiquitous in tissues. Gastrointestinal glutathione peroxidase (GPX2) is expressed exclusively in the gastrointestinal tract where it may provide an important barrier against hydroxyperoxides in dietary fat. Another isoenzyme (GPX3) is secreted into blood mainly by the kidney, also by tile mammary glands into milk. Each isoenzyme contains a selenocysteine residue and a FAD residue as prosthetic groups. The hydroxyperoxides in esterified fatty acids, especially those in phospholipids, can be reduced by phospholipid-hydroperoxide glutathione peroxidase (GPX4, EC1.11.1.12) which also uses GSH as the cosubstrate. This enzyme is expressed in the inner mitochondrial membrane and cytosol of most tissues. Thioredoxin reductase (EC1.6.4.5) is a ubiquitous NADPH-dependent selenoenzyme in cytosol which reduces both dehydroascorbate and the semidehydroascorbate radical to ascorbate.
Viral mutation: The recent recognition that low Se status of the host can increase the virulence of pathogens has thrown a new light on some manifestations of Se deficiency such as Keshan disease. An otherwise benign strain of coxsackievirus B3 was shown to cause myocarditis in Se-deficient mice. Similarly, the rate of influenza virus mutations was related to Se status of the animal host. It has been suggested that oxidative stress due to low activity of Se-containing peroxidases in the host may accelerate the emergence of mutated pathogen strains.
Thyroid hormone metabolism: Another group of selenocysteine enzymes, the thyroxine deiodinases (EC3.8.1.4), are anchored to the endoplasmic reticulum; the enzymes remove iodine from the thyroid hormones thyroxine and triiodothyronine. At least three distinct genes code for isoforms, which differ in respect to specificity and level of expression in various tissues, and at different developmental stages. Thyroxine deiodinase 2 generates the most active thyroid hormone triiodothyronine in thyroid, developing brain, anterior pituitary gland, and brown adipose tissue. The isoform 1, in contrast, inactivates thyroxin in liver and kidney and thereby ensures that hormonal activity does not accumulate unchecked. Thyroxine deiodinase 3, which is expressed in fetal tissues and placenta, preferentially removes iodine from the inner ring of thyroid hormones thereby inactivating both thyroxin and triiodothyronine. The enzyme thus protects fetal tissues from exposure to the much higher thyroid hormone concentrations in maternal circulation. Thioredoxin reductase (ECI.6.4.5) acts as a molecular chaperone which reduces inter- and intramolecular disulfide bonds ofmultimerized iodinated thyroglobulin and helps with its refolding. This may be important for the mobilization of stored thyroglobulin and subsequent release of active thyroid hormone.
Insufin metabolism: The selenium-containing thioredoxin reductase (EC1.6.4.5) is an NADPH-dependent enzyme with FAD as a prosthetic group that uses thioredoxin to reduce insulin.
Cell replication: The fragile histidine triad/dinucleoside 5',5"-Pl,P3-triphosphate (Ap3A) hydrolase (EC3.6.1.29) contains four coordinated selenium atoms and requires magnesium or manganese as cofactors. The enzyme functions as a tumor suppressor gene (Ji et al., 1999), possibly by inducing apoptosis, and through the actions of AP3A and AP4A which are thought to regulate DNA replication and signal stress responses. Through as yet unknown mechanisms Se also appears to be important for the methylation of DNA, which is known to control gene expression and thus proliferation and differentiation. The DNA in rodents with diminished Se status appears to be distinctly less methylated than DNA from Se-replete animals. The higher cancer risk of Sedeficient animals may thus be related to disrupted DNA methylation.
Other functions: The mitochondrial capsule selenoprotein (MCSP) maintains the crescent structure of sperm mitochondria. Selenoprotein P is an extraceilular heparin-binding glycoprotein with ten selenocysteine residues that may be important for selenium transport and storage, and contribute to antioxidant defense in extracellular space near endothelium. Selenoprotein W in muscle contains one selenocysteine; its function is as yet unknown. The fact that glutathione is covalently bound to Cys-36 may suggest a redox function.
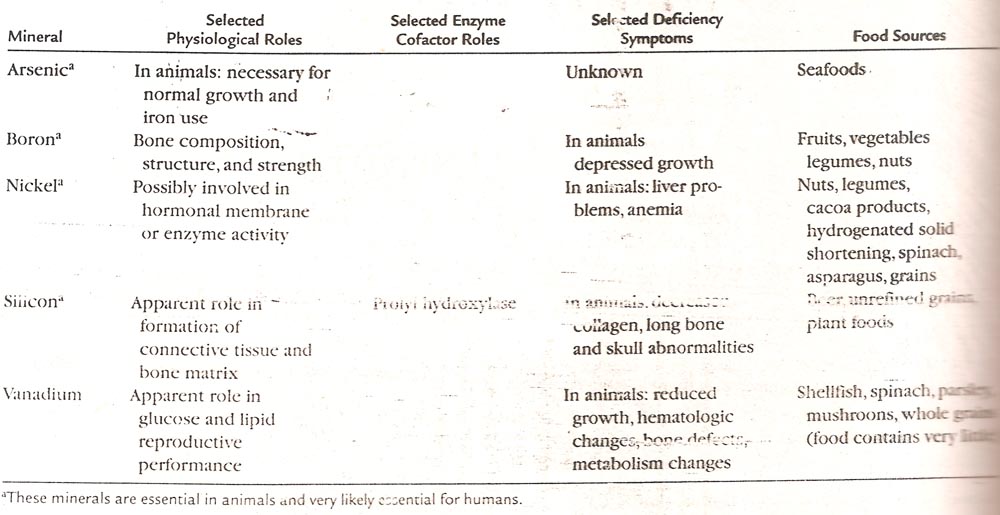
Fluorine:
Nutritional summary
Function: Fluoride increases resistance of teeth to dental caries, and may help retain bone minerals in old age.
Requirements: Fluoride is not an essential mineral, but daily intakes of 0.05 mg/kg body weight greatly reduce the risk of dental caries in children and adults. Topical applications (toothpaste, tooth sealants, mouthwash, and dental floss) also promote the re-mineralization of teeth.
Food sources: Major dietary sources of fluoride are naturally or artificially fluoridated water, seafood, tea, and unintentional ingestion of dental products. Typical daily intakes by adults in the US have been estimated to range from 1.2 to 2.4 mg.
Defidency: Contrary to earlier assumptions, fluoride has not been shown to be an essential trace mineral. Benefits for dental and bone health, however, exist under most conditions.
Excessive intake: Daily doses of 0.1 mg/kg during tooth development, which is only twice the recommended intake, cause mild to moderate tooth mottling (brown spotty discoloration) in children, but not in adults. Acute poisoning with nausea, vomiting, diarrhea, salivation, sweating, headache, and generalized weakness, hypotension, renal failure, severe hypocalcemia and hyperkalemia, cardiac arrhythmia, coma, and death may occur with less than 5 mg/kg in children, a dose that could result from ingesting fluoridated toothpaste. Calcium solutions including milk can be used to slow absorption in an emergency situation. Long-term exposure to unusually large amounts of fluoride (20 mg/d) may cause abnormal bone and ligament calcification.
Dietary sources
Major sources are fluoridated water and dietary supplements, toothpaste, seafood, tea. Most toothpastes contain 1 mg/g, but prescription strength products may contain as much as 5 mg/g. The water in some world regions naturally has fluoride content well in excess of 1 mg/l. Bottled Vichy water contains 5 mg/l, most other bottled waters contain much less. Water purification systems, with the exception of reverse osmosis and deionization systems, remove little fluoride from water.
Function
Bone and joints: Fluoride stimulates new bone growth. Whether lowdose fluoride intakes reduce bone mineral loss in older people remains a contentiously debated issue, because the newly formed bone may have a less optimal structure and be less resistant to fracture. A very high tissue concentration of fluoride (2 mmol/l) inhibits acid phosphatase in chondrocytes and bone matrix. Fluoride has also been suggested to inhibit a particular phosphotyrosine phosphatase in osteoblasts. This could promote tyrosine phosphorylation in key signaling proteins of the mitogenic transduction pathway and growth factor-dependent proliferation of bone cells.
Teeth: Some fluoride is firmly bound to tooth minerals and forms the hard composite mineral fluorapatite. Topical application of fluorides can accelerate the re-mineralization of teeth. Fluoride also affects the ability of bacteria to attach through glucan-binding lectins and may reduce acid production by bacteria.
High intake: Excessive intake causes a characteristically mottled appearance of teeth, but only during tooth development in children. It has been suggested that high natural fluoride exposure (several milligrams per day) increases the risk of kidney stone formation.
Copper:
Nutritional summary
Function: Copper is essential for energy metabolism (cellular respiration), brain function (neurotransmitter regulation), soft tissues and bone (collagen synthesis), for nutrient metabolism (especially iron), and for antioxidant defense against free radicals (free radicals increase the risk of cancer and cardiovascular disease).
Requirements: Adults should get 0.9 mg/day (Food and Nutrition Board, Institute of Medicine, 2001). Smoking, strenuous exercise, heat, infections, and injuries each may increase needs by 50% or more.
Food sources: The best food sources of copper include liver, shellfish, nuts, and seeds. Most other foods provide smaller amounts that in combination usually are enough to meet normal needs. People who follow the food guide pyramid recommendations ensure their adequate copper intake.
Deficiency: Deficiency is very unlikely to occur except in people with very rare genetic disorders or during prolonged starvation. Symptoms include anemia, low white cell count, accelerated bone mineral loss, and increased blood pressure and cholesterol levels. If intakes are low, stores last only a few weeks.
Excessive intake: Daily doses in excess of 10 mg (typically from supplements or from contaminated stored or well water) can cause nausea, vomiting, abdominal cramps, diarrhea, and liver damage (especially in infants). Higher doses may lead to coma and death.
Dietary sources
Exceptionally rich sources of copper include liver (45 mg/kg), kidney (35-50 mg/kg), oysters (7.4 mg/kg), walnuts (16 mg/kg) and other nuts and seeds. Copper-lined pipes or vessels do not increase copper content of their contents significantly unless exposed to acids. Average daily intake of American adults is about 1.6 mg in males and 1.2 mg in females (Food and Nutrition Board, Institute of Medicine, 2001).
Function
Iron metabolism: Ferroxidase (ceruloplasmin, iron (ll):oxygen oxidoreductase, EC 1.16.3.1 ) contains 6-7 copper atoms that can be transferred to other tissues. This multifunction protein scavenges free radicals and oxidizes iron bound to transferrin. This latter function is important, but not essential for mobilization of iron from stores (Linder et al., 1999). Excess ascorbate may interfere with ceruloplasmin activity. Hephaestin, a copper-containing metalloprotein, is present mainly in the basolateral membrane of intestinal villi and is thought to facilitate iron exit from enterocytes. The structure of this metalloprotein closely resembles that of ceruloplasmin.
Hormone metabolism: Copper is a component of several enzymes that participate in the metabolism of catecholamines and other compounds with regulatory activity. Monophenol monooxygenase (tyrosinase, EC1.14.18.1) catalyzes the first step of dopamine and dopaquinone synthesis. Dopamine-beta-monooxygenase (dopamine hydroxylase, EC 1.14.17.1, requires ascorbate and contains autocatalytically generated topaquinone) is needed for noradrenaline synthesis, particularly in brain. Copper-containing amine oxidases (EC1.4.3.6, includes amiloride-sensitive amine oxidase, retina-specific copper amine oxidase, and membrane copper amine oxidase, which is identical with vascular adhesion protein-l) release histamine and inactivate polyamines (putrescine, spermine, spermidine). The amine oxidases contain topaquinone as a cofactor. Several peptide hormones, including thyroid-releasing hormone (TRH), corticosteroidreleasing hormone (CRF), gonadotropin-releasing hormone (GnRH), neuropeptide Y, endorphins, gastrin, pancreatic polypeptide, atrial natriuretic factor, arginine vasopressin, alpha-melanotropin, become active only after removal of the terminal glycine residue by peptidylglycine monooxygenase (peptidyl alpha-amidating enzyme, EC 1.14.17.3, also requires ascorbate).
Energy metabolism: Cytochrome c oxidase (EC 1.9.3.1 ) catalyzes the rate-limiting terminal step of oxidative phosphorylation at the inner mitochondrial membrane. The copper in subunit 2 of the complex transfers electrons from cytochrome c to the copper and iron-containing center of the catalytic subunit 1. A vectorial Bohr mechanism (conformation shift due to oxygenation change) moves reduced protons from the inner aqueous space through the mitochondrial membrane and the heme-copper oxidases then release them into the external space. This proton translocation builds up a proton gradient across the inner mitochondrial membrane that drives the synthesis of ATE
Antioxidation: In addition to the above-mentioned function as a component of the free radical scavenger ceruloplasmin, copper is also an essential constituent of several superoxide dismutases (ECI. 15.1.1). Different forms require zinc, iron or manganese for the second metal-binding site and have distinct functions. The enzymes, which are present both in cytosol and the extracellular space, dissipate highly reactive and therefore toxic superoxide radicals by converting them to hydrogen peroxide. It should be emphasized that free copper ions greatly promote the formation of oxygen free radicals in a variety of reactions. Excess copper therefore increases oxidative damage of DNA and proteins.
Connective tissue: The maturation of the structural proteins in bone, cartilage, blood vessels and other tissues depends on the action of protein-lysine 6-oxidase (EC1.4.3.13). This copper enzyme catalyzes the hydroxylation of specific lysine residues in collagen and elastin molecules and thereby initiates the crosslinking of individual strands. Four additional lysyl oxidase-like proteins (LOXLs) have been identified so far. Functions are very diverse and include cell adhesion and motility, tumor suppression, and regulation of cellular senescence.
Blood coagulation: Copper plays a significant role in normal blood clotting through its interaction with coagulation factors V and VIII. Factor V contains a specific (type Ii) copper-binding site. The interaction of the heavy and light chain components of factor VIII stabilizes the complex and maintains its activity.
Gene expression: It is likely that some transcriptional events involve copper. Several copper-containing transcription factors have been identitied in yeast, including Ace lp and Mac lp, but it is unclear whether there are human homologs. It is of interest in this respect that copper deficiency decreases transcription of the IL-2 gene in human mononuclear cells and lymphocytes.
Manganese:
Nutritional summary
Function: Manganese is a cofactor for many enzymes with importance for carbohydrate metabolism, protein digestion and metabolism, biotin function, cartilage regeneration, and free radical defense.
Food sources: Black tea, nuts, grains, sweet potato, and spinach each provide at least 1/6 of adequate daily intake per serving.
Requirements: Daily intakes of 1.8 mg for women, and of 2.3 mg for men, are thought to be safe and adequate (Food and Nutition Board, Institute of Medicine, 2001 ).
Deficiency: Low intakes cause growth retardation in children. Decreased fertility, higher susceptibility to seizures, and bone fractures are less certain consequences of deficiency.
Excessive intake: No toxic effects of ingested food grade manganese have been reported even at many times typical intakes. Very high intakes or non-dietary exposure induce tremor, delayed movements (akinesia), and rigidity due to extrapyramidal damage. Some derivatives used as gasoline additives and for other non-food purposes are toxic.
Dietary and other sources
Good sources (more than one-sixth of adequate intake per serving) include black tea, red wine, pecans, peanuts, pineapple, oatmeal, shredded wheat, raisin bran cereal, beans, rice, sweet potato, whole wheat, and spinach. Blueberry juice contains 21microg/1. Water usually contributes only a few micrograms or less per day. Inhaled manganese dust particles, often due to workplace exposure, are expectorated and secondarily swallowed to some degree. Typical daily manganese intakes of American women are around 1.9 mg; men's daily intakes are around 2.4mg. Median intakes in the Total Diet Study gave comparable results (Food and Nutrition Board, Institute of Medicine, 2001). Similar average intakes (2.2 mg/day) were also observed in an Indian population. Manganese is unusual, because it can be taken up by olfactory neurons and transported directly to the brain.
Function
Many enzymes that use a divalent cation as a cofactor are not very specific in metal ion requirement. Manganese often can substitute for magnesium, sometimes for zinc. Typical examples are the cholesterol synthesis enzyme geranyltranstransferase (farnesyl pyrophosphate synthetase, EC2.5.1.10), and the key enzyme of lipid metabolism, phosphatidyicholine synthase (EC2.7.8.24), which work equally well with magnesium and manganese. The following will focus only on functions that specifically require manganese.
Vitamin B 72 activation: Ingested vitamin B 12 has to be metabolically processed before it can serve as a cofactor with the three known B12-dependent enzymes. Cob(1)alamin adenosyltransferase (EC2.5.1.17) produces the form of vitamin B I2 that is a cofactor of 5-methyltetrahydrofolate-homocysteine S-methyltransferase (EC2.1. !. 13) and of L-beta-leucine aminomutase (EC5.4.3.7). Since cob(I)alamin adenosyltransferase has been characterized only in microorganisms so far, considerable uncertainties about its divalent metal requirements remain.
Free radical control: Manganese-containing superoxide dismutase (ECI.15.1.1) is a critical component of the defenses against the free radicals that are a side product of oxidative phosphorylation and other oxidative reactions in mitochondria. Free radicals also are used by immune cells as a corrosive agent for the destruction of infectious agents. The importance of the protective function ofMn superoxide dismutase is illustrated by the fact that it is induced when monocytes become activated by bacterial endotoxin and increase free radical production.
Apoptosis: A strictly manganese-dependent enzyme, ATM protein kinase (no EC number assigned) phosphorylates p53, PHAS-I, RPA32 and Chk2 in response to cell damage and induces thereby cell cycle arrest at the GI/S and G2/M stages. The oncogene p53 participates in the control of programmed cell death (apoptosis). ATM protein kinase is defective in the genetically inherited condition ataxia teleangiectasia (hence the acronym ATM), which is characterized by high cancer incidence at young adult age, immune dysfunction, and loss of coordination of movements (ataxia). Manganese may also be important for the function of DNA-protein kinases related to ATM protein kinase, possibly through modifying the type of reaction they catalyze.
Nitrogen metabolism: Arginase (EC3.5.3.1) is the final enzyme for urea synthesis. Both the cytoplasmic and mitochondrial isoforms require manganese as a cofactor. While arginase is not involved in nitric oxide signaling, it is of note that manganese potentiates nitric oxide production by microglia.
Peptide hydrolysis: The ubiquitous cytosolic Xaa-Pro dipeptidase (prolidase, EC3.4.13.9)
and the bradykinin-degrading Xaa-Pro aminopeptidase (EC3.4.11.9) have an absolute manganese requirement. The latter is important for the degradation of bradykinin. The protein encoded by the gene AMPL (cytosol aminopeptidase) exemplifies a more complex picture. This protein has two metal-binding sites, of which the first one always contains zinc. When the second binding site contains zinc, the protein has the catalytic activity ofleucyl aminopeptidase (EC3.4.1 I. 1) and preferentially cleaves an N-terminal leucine from peptides. If the second metal-binding site is occupied by manganese, on the other hand, the protein behaves as prolyl aminopeptidase (EC3.4.11.5) and cleaves the N-terminal proline from a peptide. In combination these activities are crucially important for the intracellular processing and turnover of proteins.
Glycolysis and gluconeogenesis: The bulk of energy-providing nutrients is converted into acetyI-CoA, which is then utilized through the Krebs cycle. Acetyl-CoA can only be fed into the cycle through condensation with oxaloacetate. Supplies of this indispensable intermediate are drained when significant gluconeogenesis or net-synthesis of aspartate and asparagine occurs. The manganese (and biotin)-dependent enzyme pyruvate carboxylase (EC6.4.1.1) is crucial for replenishing oxaloacetate supplies, because it catalyzes the only reaction that can synthesize it without drawing on preformed exogenous Krebs cycle intermediates or amino acids. Each subunit of the pyruvate carboxylase homotetramer contains one tightly bound manganese ion. The enzyme may also contain a mixture of manganese and magnesium. The mitochondrial form of the key enzyme for gluconeogenesis from lactate and proteins, phosphoenoipyruvate carboxykinase (PEPCK, EC4.1.1.32), also is manganese-dependent.
Cartilage formation: Several glycosyl transferases with a stringent Mn(II) requirement participate in the formation and maintenance of cartilage and other connective tissues. N-acetylneuraminylgalactosylglucosylceramide beta- 1,4-N-acetylgalactosaminyltransferase
(EC2.4.1.165) and polypeptide N-acetylgalactosaminyltransferase (EC2. 4.1.41) process fetuine, mucin, and other proteoglycans. Synthesis of the cartilage glycoprotein chondroitin is another process that relies heavily on manganese-dependent enzymes. Xylosylprotein 4-beta-galactosyltransferase (EC2.4.1.133), galactosylxylosylprotein 3-beta-galactosyltransferase (EC2.4.1.134 ), and galactosylgalactosyixylosylprotein 3-beta-glucuronosyitransferase (EC2.4.1.135) add the second, third, and fourth of the four sugar linkage side chain (3-beta-D-glucuronosyl-3-beta-Dgalactosyl -4-beta-D-galactosyl-O-beta-D-xylosyl) in chondroitin sulfate and other proteoglycans, especially in fibroblasts. Glucuronyl galactosyl proteoglycan beta-l,4-N-acetyl galactosaminyl transferase (EC2.4.1.174) adds N-acetyl-D-galactosamine to an acidic chondroitin sulfate side chain. Manganese-dependent glycosyl transferases also are crucial for the extension ofceramide side chains. Enzymes with such activities include beta-galactosyl-N-acetylglucos aminylgalactosyl-glucosyl ceramide beta-l,3-acetyl glucosaminy ltransferase (EC2.4.1.163), galactosyl- N -acety lglucos aminyl galactosyl-glucosy lceramide beta-l,6-N-acetylglucos aminy ltransferase (EC 2.4.1.164), and N-acetyl neuraminyl galactosyl glucosyl ceramide beta-l,4-N-acetylgalactosaminyltransferase (EC2.4.1.165).
Sulfate metabolism: Sulfate is released from its activated form by manganese-dependent
phosphoadenylylsulfatase (EC3.6.2.2).
Toxic effects: Accumulation of manganese in brain, often due to liver disease (impaired excretion), parenteral feeding or industrial exposure, increases dopamine turnover. Depletion of dopaminergic brain regions may then be the cause of a manganeseinduced Parkinson disease-like syndrome with muscle tremors, delayed movement (akinesia) and rigidity.
Iodine:
Nutritional summary
Function: Iodine is an essential component of the thyroid hormones which have profound impact on development, growth, and metabolism.
Food sources: Good sources are fish, shellfish, seaweed, iodized salt, and fortified foods. Other foods provide variable amounts depending on the soil iodine content of their origin.
Requirements: Adults should get 150 microg/d; slightly more is needed during pregnancy and lactation.
Deficiency: A lack of iodine may cause spontaneous abortion and birth defects; it impairs irreversibly brain and physical development in the fetus and young child, causing from mild to severe mental retardation, sometimes also hearing loss or paralysis of the legs. Deficiency in older children and adults stimulates excessive growth of the thyroid gland (goiter), slows metabolic rate, mental and cardiac function, and induces a feeling of fatigue and cold intolerance.
Excessive intake: Increased iodine intakes can promote thyroid hormone production in people with excessive function of the thyroid gland (autonomous hyperthyroidism). In healthy people, intakes above requirements (up to 2000 microg/d) will not confer any benefit, but appear to cause no harm. Chronic intake of several milligrams per day may disrupt thyroid function.
Dietary sources
Foods contain iodine mainly as iodide (1-), iodate (IO 3 ), or thyroxine. Best iodine sources are marine fish, shellfish, seaweed, sea salt; iodized salt contains 76 microg iodide/g, the iodide content of sea water is 50-60 microg/I. Bread may contain iodate as a dough improver, and dairy foods may contain iodine from udder antiseptics. Unsupplemented iodine content of foods closely correlates with the concentration in soil.
Plants from iodine-sufficient soil contain around 1 microg iodine/g dry weight. Plants from areas with iodine-depleted soils (old mountain ranges and coastal areas) contain only a fraction of that amount. Drinking water contains less than 2 microg/I in iodine-deficient areas. Typical iodine intake in the US is 100-150 microg/d.
Function
Classic role of thyroid hormones: Thyroid hormones are critically important for the fetus and young infant by affecting development and maturation of nervous system, skeletal muscle, and lungs through as yet incompletely understood mechanisms. At all life stages thyroid hormones promote intermediary metabolism; disruption of thyroid hormone production slows oxidation, lowers body temperature, and may evolve into a life-threatening condition. About 60microg iodine/day are used for hormone production. If slightly less than this amount is available, goiter (enlarged thyroid gland) may develop. A reduction of iodine content of the thyroid below 0.1% usually results in hyperplastic changes. Enlargement of the thyroid can also be related to a disruption of thyroid hormone synthesis by consumption of thioisocyanates from cabbage and other cruciferous vegetables.
Non-genomic thyroid hormone actions: Thyroid hormones also act without changing gene expression. They influence the transport of sodium, calcium, glucose and other solutes, modulate the activities of protein kinase C, cAMP-dependent protein kinase and other kinases, affect glycolysis enzymes such as pyruvate kinase M2, oxidative phosphorylation, and regulate skeletal and vascular smooth muscle action.
Cobalt:
Nutritional summary
Function: An enzyme with cobalt (Co) as an essential cofactor is involved in the regulation of translation; Co also may be a constituent of an oxygen sensor.
Requirements: It is not known what intakes are needed to maintain optimal health.
Food sources: The Co content (other than cobalamin) of foods is not well documented. Deficiency: It is not known whether there are any untoward effects of low intakes or what the consequences of Co deficiency in humans might be.
Excessive intake: Pericardiomyopathy occurred with chronic intakes of 6-8 mg/d in beer. More common consequences of acute intake of 100 mg or more include hypotension, nausea and vomiting, diarrhea, loss of appetite, tinnitus, acoustic nerve damage, hypothyreosis and goiter, hyperlipidemia, and xanthomatosis. High tissue levels of divalent Co may be associated with genotoxic and carcinogenic effects.
Dietary sources
The non-corrinoid Co content of individual foods is not well documented. Investigators in the UK found significant amounts in nuts (9 microg/100g), sugars and preserves (3 microg/100 g), potatoes (9 microg/100 g), and bread (9 microg/100 g). Esti mates from studies in various countries point to total Co intakes (including Co covalently bound to cobalamins and other corrinoids) of about 11 micrograms. The amounts of Co that can be leached by food acids from porcelaine dishes and thus become available for ingestion appear to be limited to a few micrograms.
Function
Gene translation: Methionine aminopeptidase (EC3.4.11.18) is the only enzyme in humans which has a firmly established cobalt requirement. This enzyme plays an important role in translational regulation by preventing the phosphorylation of the alpha subunit of initiation factor-2.
Non-specific enzyme cofactor: While there is no evidence that Co is a required or otherwise physiologically significant cofactor of enzymes other than methionine aminopeptidase (EC3.4. l 1.18), Co can substitute for other divalent cations in a number of enzymes. In some instances, such as mevalonate kinase (EC2.7.1.36), it may be a better activator than magnesium, manganese, or calcium.
Oxygenation sensing. A heine-containing oxygen sensor (hypoxia-inducible factor l, HIF-I ), in which iron can be substituted by Co or nickel, stimulates erythropoietin production. This sensor appears to be a multi-subunit flavohemoprotein complex with b5 and b5 reductase domains and an NAD(P)H oxidase capable of generating peroxide and reactive oxygen intermediates which serve as signaling molecules. The physiological significance of Co for oxygen sensing remains in doubt. It may be worth noting, however, that Co has been used in the past for the (controversial) treatment of anemia as a stimulant of hernatopoiesis.
Chromium:
Nutritional summary
Function: A peptide-chromium(lll) complex increases insulin action. Chromium alone binds to nuclear DNA and increases the number of initiation sites for RNA synthesis.
Sources: Nuts, whole grains, yeast, cheese.
Requirements: Recommendations for adequate intakes have been set at 35 microg/d for younger healthy men, and 25 microg/d for healthy women (Food and Nutrition Board, 2002), slightly less for men and women over the age of 50.
Deficiency: Low intakes (less than 20 microg/d) may decrease the cellular response to insulin and slow the utilization of stored fat.
Excessive intake: Chromium(Vl), but not the biologically active chromium(Ill), may be carcinogenic even in small amounts. Chromium picolinate may promote oxygen free radical formation.
Dietary sources
Good dietary chromium sources include yeast, whole grain and wheat germ, cereals, nuts, cheese, and organ meats. Human milk has been reported to contain 24microg/l, and infant formulas have similar concentrations. Synthetic chromium picolinate is a commonly used form in dietary supplements. Both natural and supplement sources contain almost exclusively chromium(lll). There is great uncertainty about average intakes of American adults, with estimates ranging from below 20 to over 40microg/d.
Function
Insulin-receptor activity: The chromium-containing peptide chromodulin binds to the insulin-activated insulin receptor and optimizes its receptor kinase activity. Thus, chrornodulin potentiates the ability of insulin to promote oxidative glucose metabolism and fatty acid synthesis from glucose in adipocytes. Raising chromium intakes from low to adequate levels is likely to restore insulin receptor function, if this was impaired due to the deficiency. It is not clear, however, whether chrornium-replete diabetics will benefit from additional intakes.
DNA transcription: Chromium binding to DNA appears to increase the number of initiation sites, thereby promoting RNA synthesis.
Other effects: Hexavalent(Vl) chromium compounds have been found to induce rnalignant cell growth in animal models; solubility of the compounds was an important determinant of carcinogenic potential. Most epidemiological studies have focused on the increased risk for nasal, throat, and bronchial cancer following workrelated (dust) exposure; data on low-level oral exposure are lacking. An anecdotal report linking renal failure to long-term chromium picolinate ingestion has been disputed. It has been argued that chromiurn picolinate may form redox-active complexes with ascorbate and thiols. In vitro experiments demonstrate that mixtures of chromium picolinate and ascorbate can indeed induce the formation of hydroxyl radicals and lead to DNA damage.
Bromine:
Nutritional summary
Function: Bromine (Br) is used by eosinophilic leukocytes for immune defense.
Food sources: Significant contributors to dietary intake grains, nuts, sea salt, seafood, and bread.
Requirements: No requirements have been established. Intakes around 8 mg/day appear to be adequate.
Deficiency: The consequences of chronically low intake are uncertain; growth retardation and insomnia have been suggested. People on chronic hemodialysis may be at increased risk.
Excessive intake: High acute exposure, such as from swallowing excessively brominated pool water during swimming, or from inhalation, may cause bronchospasm, headache, gastrointestinal disturbances, fatigue, reduced exercise tolerance, and myalgia.
Dietary and other sources
Grains, nuts, seafood and sea salt are significant dietary sources. Brominated flour is sometimes used for bread and other baked goods. Average Br intake in young Dutch adults has been reported to be 8 mg/day. Occasional sources may be Br-based sanitizing agents and brominated swimmingpool water.
Function
Immune defense: A specific peroxidase (ECI.I 1.1.7) in the cytoplasmic granules of eosinophils uses Br to generate a halogenating oxidant, which bromihates tyrosines and other amino acids in proteins. This hemeenzyme is structurally distinct from myeloperoxidase in neutrophils, myelocytes, and macrophages. While other peroxidases generate hypochlorous acid (HOCI) as an oxidative reactant, eosinophil peroxidase produces hypobromous acid in an analogous reaction:
Br- +H202 +H+<-->HOBr +H20
HOBr potently brominates the deoxycytidine moieties of DNA and thereby disrupts replication of" invading parasites. Additional corrosive reactants are generated by both eosinophils and neutrophils, including the interhalogen CIBr as shown in the following reaction scheme:
HOCI +Br- +H+~-->BrCI +H20
This very volatile interhalogen is readily hydrolyzed:
BrCI +H20~-->HOBr +CI- +H +
Taurine can be brominated to the corrosive oxidant N-bromotaurine by reacting with hypobromous acid or with hypochlorous acid in the presence of` bromide. N-bromotaurine brominates the deoxycytidine in DNA at neutral pH, while N-chlorotaurine can do this only in an acid environment. These reactive molecules can also attack tissue proteins and DNA. Prolonged and excessive production due to inflammation has been tentatively linked to cancer and atherosclerosis.
Thyroid function: Br may compete with iodine for transport into the thyroid gland and thereby mildly inhibit thyroid function.
S1eep: Low Br status has been related to insomnia in patients whose hemodialysis constantly removes a significant portion of the bromide in blood.
Nickel:
Nutritional summary
Function: Cell membrane stability and production of some hormones may be influenced by nickel availability. Nickel also is a cofactor of various microbial enzymes. Nickel intake might, therefore, influence microbial action in human intestines.
Food sources: Grains, chocolate, nuts and dried legumes are good sources of nickel. Americans typically get about 300 microg nickel per day from water and food.
Requirements: Less than 100 microg/d appears to be needed by healthy adults.
Deficiency: Low availability impairs reproduction and growth in some mammals and may impair iron, copper, and zinc metabolism.
Excessive intake: Chronic intakes of several milligrams per day may cause oxidative damage to DNA and cell structures, interfere with hormonal function, promote excessive zinc and iron storage, and lead to magnesium deficiency.
Dietary sources
Significant amounts of nickel are consumed with chocolate, nuts, oatmeal and other grains, dried beans and peas. Americans take in between 100 and 300micrograms of nickel per day with food and drinking water.
Function
A specific function of nickel in humans is not known. Nickel stimulates erythropoietin production, possibly through a heme-containing sensor in which iron can be substituted by nickel or cobalt. The sensor is a multi-subunit assembly containing an NAD(P)H oxidase capable of generating peroxide and reactive oxygen intermediates, which serve as signaling molecules.
Nickel is a cofactor for a few bacterial enzymes, mainly those involved in the utilization of hydrogen. Nickel intake may thus influence human health through its importance for intestinal flora. Nickel-dependent bacterial enzymes include carbon-monoxide dehydrogenase (EC 1.2.99.2), hydrogen dehydrogenase (EC 1.12.1.2), coenzyme F420 hydrogenase (EC 1.12.99.1), hydrogenase (EC 1.18.99.1), urease (EC 3.5.1.5), methylcoenzyme M reductase (no EC number assigned), nickel-superoxide dismutase (no EC number assigned), lactoylglutathione lyase (glyoxalase I, EC 4.4.1.5), and cis-trans isomerase (no EC number assigned).
Macrominerals

Microminerals
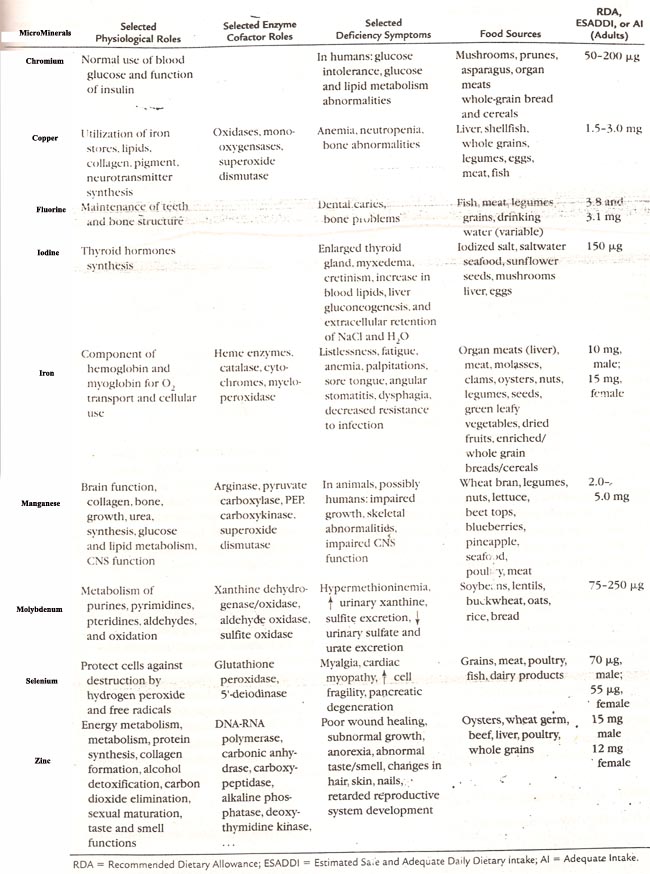
Ultratrace elements