Nutrition and immunity
Malnutrition has an adverse impact on cell-mediated, secretory and humoral immunity, as well as on non-specific host defences. Although most clinical observations concerning this problem have been made in patients with generalized malnutrition, deficiencies or excess of single nutrients can also cause acquired functional derangements. Acquired anergy can generally be reversed when malnutrition is corrected. Further, secondary nutritional derangements tend to be less severe than primary congenital defects of an immune or non-specific defence component. Unlike the relative resistance to infection seen in patients with uncomplicated starvation, cachexia associated with severe disease, malignancy, or trauma is typically accompanied by superimposed infections, especially due to opportunistic microorganisms that are normally of low pathogenicity. The hypermetabolism of most severe diseases is accompanied by complex biochemical responses, increased energy generation, and an inability to conserve amino acid stores. Secondary derangements in the function of defensive mechanisms and immune responses may then emerge rapidly. Further, loss of body nutrients during primary infection increases the susceptibility of a patient to secondary infections. Chronically malnourished patients often exhibit an infection or infestation as a coexisting problem.
Nutritional effects on immune mechanisms
Although generalized malnutrition can affect all aspects of host immunity, the impact if greatest on T cell functions and cell-mediated immunity and smallest on B cell functions and humoral immunity. Effects on secretory immunity fall in between.
Slight leukopenia, together with relative lymphocytosis was found in patients suffering from eating disorders. In fact, plasma concentrations of IgM, IgG, IgA, IgD, and IgE may all be greater than normal in malnourished infants, and blocking antibodies and antibodies against food antigens may increase. The humoral immunity, judged by serum IgA concentration, was different in adolescent females suffering from obesity or anorexia nervosa; obese patients showed the highest values.
Apparently paradoxical increases in plasma Ig values during malnutrition have not been explained with certainty, but may involve the presence of infectious or parasitic diseases. Children of developing countries tend to experience a continuing heavy exposure to multiple antigens. Vitamin A supplementation results in an increased IgG response to the wild measles virus. During severe generalized malnutrition, lymphoid tissue atrophy develops primarily in the T cell areas and circulating T cell numbers decline. Loss of delayed dermal hypersensitivity to previously encountered antigens is typical and de novo dermal sensitization responses are impaired. In vitro responsiveness to mitogens and antigens is impaired in T cells from patients with generalized protein-energy malnutrition. Host-versus-graft reactions are delayed.
Protein undernutrition has been shown to suppress significantly the secretion of lysozyme into tears. Lysozyme levels in tears were significantly lower (50% less) in moderately malnourished grade II children than in normal children. The synthesis and secretion into tears of other locally produced proteins, slgA and amylase were also impaired. Decreased activity of lysozyme has also been observed in leukocytes of children with protein-calorie malnutrition (PCM). Reduced concentrations of slgA and, to lesser extent, lysozyme, are potentially important in the defence of mucosal surfaces. The lower levels differ significantly from those serum proteins, such as total protein, IgG, aminopeptidase, and albumin, levels of which in tears were not influenced by nutritional status. This indicates that the reduced levels of slgA, amylase and lysozyme were not due to reduced volume of secretion in malnourished children, as has been suggested by experimental studies on saliva in malnourished rats. It appears that the mechanism involves impairment in the local synthesis and/or secretion of these proteins. These observations have been confirmed by studying parotid saliva during the renutrition of children with kwashiorkor or marasmic malnutrition. Saliva flow rate (millimetre per minute) increased about 50% during a 4-week renutrition with a high protein diet. Therefore, the non-specific immunity due to the washing effects of secretions was significantly increased. Decreased total salivary IgA levels in children with acquired immune deficiency syndrome (AIDS), with a compromised nutritional status were reported.
Essential ammo acid deficiency and immune response
Although experimentation in protein malnutrition is easier to conduct, specific essential amino acid deficiency may be of more importance and significance to human conditions of malnutrition. Is has long been realized that many proteins, especially of plant origin, are deficient in certain essential amino acids. For example, wheat gluten and maize protein are limited in lysine, and soya protein is limited in methionine. Malnutrition often occurs when these foods are consumed as sole sources of protein in the diet.
The administration of a low-quality dietary protein (cooked maize flour) from weaning onwards provokes damage in the rat mesenteric lymph node due to the lack of B—T cell cooperation. This diet would produce a disturbance in zinc metabolism concomitant to, or as a consequence of, the state induced by the administration of this type of protein. The wheat-based diet was limited in lysine, methionine, threonine and isoleucine but adequate in other essential amino acids and nutrients. The rice-based diet was limited in methionine and threonine. Furthermore, it is theoretically possible to acquire essential amino acid malnutrition even when good quality protein is consumed in limited quality. The level of each essential amino acid needed for optimal growth of mice has been determined. For example, mice fed an 8% casein diet consume inadequate levels of methionine, but the levels of other essential amino acids were considered adequate. The dietary level of protein and quality of the dietary protein are both important with respect to the resistance of infection. Mice fed with 20% gluten diet were more susceptible to infection than mice fed with 20% casein diet. The animals fed the casein diet were less susceptible to infection with Salmonella enteritidis. Animals fed a lysine-deficient diet were more susceptible to Bacillus anthracis, the causative agent of anthrax. The decreased resistance remained even after supplementation of lysine in the diet. An increased susceptibility to S. typhimurium has been demonstrated in mice fed a hystidine- or threonine-limited diet.
Nutritional deficiences and mucosal immune defence systems in animals
Marginal protein malnutrition in a nutritionally well-defined animal model system confirmed the observations made in humans that protein maltnutrition suppressed slgA. slgA was significantly lower in the tears and vaginal secretions of marginally malnourished guinea pigs fed an 8% protein diet. IgG, which was the predominant immunoglobulin in vaginal secretions, was not affected by nutritional stress. It would be expected that mechanisms of host defence against a microbe infecting a mucosal surface should be related to levels of secretory antibodies. A significant depression of slgA levels was found in both tears and genital secretions of guinea pigs suffering marginal and severe protein deprivation. slgA in both tear and genital secretions of animals fed a 9% protein diet (marginally malnourished) were significantly lower than control levels both before and after infection with guinea pig inclusions conjunctivitis (GPIC). Post-infection slgA levels in tears of these marginally malnourished animals reached only 69% of post-infection control levels, and the genital secretion slgA levels averaged only 61% of controls. In the severely malnourished group, slgA reached only 60% of control levels in tears and 59% of controls in genital secretions. In addition, malnourished groups and their controls showed significant increases in slgA for both tears and genital secretions after GPIC infection, suggesting that the organism did elicit a secretory antibody response in all animals studied. slgA anti-GPIC antibody was produced in tears of all but the most severely malnourished guinea pigs after infection with GPIC.
As in human secretions, the levels of IgG were not affected in genital secretions, in which IgG is the principal immunoglobulin. The gradual increase in slgA levels in the weaning animals with increasing age suggests that a slgA development process may be affected by nutritional stress. Total protein in the secretions of both severely malnourished groups and their controls increased significantly after infection, possibly reflecting the IgA response, since IgG levels in genital secretions and aminopeptidase in both tears and genital secretions did not increase after infection. IgG should play a significant role in mucosal immunity, as it is the second secretory immunoglubulin by concentration in most secretions. The effects of protein malnutrition in its concentration in saliva confirm the study of IgG in vaginal secretions and human secretions. Salivary IgG levels in moderately protein-malnourished rats did not decrease, but their amylase levels did. Amylase is largely locally synthesized, but much of IgG comes from the serum into saliva by transudation. Serum IgG levels are generally not suppressed by protein malnutrition. These animal and human studies strongly suggest that severe protein or calorie deficiencies do not significantly alter total IgG in secretions. In trying to understand how protein deficiency suppresses slgA, they tested the hypothesis that T cell functions were suppressed. As the thymus is known to be suppressed by protein deficiency, they treated protein-malnourished young mice with a thymus hormone preparation, thymosin fraction V. However they found that there was no apparent effect on the quantity of slgA in the intestinal secretions of these mice.
Also, the effect of severe protein deficiency in Wistar rats at weaning has been studied in bone marrow, which is a primary lymphoid organ, and which some authors consider to be associated with the mucosal immune system. Cytogenetic studies in bone marrow cells from Wistar rats with protein malnutrition have principally shown: (a) decreased number of viable bone marrow cells; (b) diminished percentage of mitosis; and (c) severe alterations in the percentage of 3, 11 and 12 chromosome pairs bearing nucleolar organizing regions (NORs), that reflects a poor ribosomal gene activity showing that RNA transcription is affected but (d) a 20% casein diet administered for five days reversed this situation.
It is well-known that T cells can be stimulated by LPS from Escherichia coli or other bacterial infections. Nutritional deficits, excesses, or imbalances influence host resistance in a diverse manner. Although it is traditionally thought that malnutrition impairs host resistance, such an assumption is not always true. Hundreds of reported studies of infection in malnourished human or animal hosts were evaluated and interactions in which the presence of malnutrition made the infection seem more severe were classified as antagonistic. In virtually all studies in humans, malnutrition either contributed to a synergistic increase in disease severity or had no demonstrable effect. The same synergistic trend was evident in most experimental bacterial infections in animals. In contrast, experimental viral infections showed an antagonistic interaction about as frequently as a synergistic one, and parasitic infestations fell about midway between bacterial and viral infections.
Although the fundamental mechanisms that could account for synergistic or antagonistic interactions have not been identified with certainty, the nutritional status of the host can influence the metabolic processes of an invading microorganism as well as those of the host. Because most bacteria possess their own replicating machinery and have relatively simple nutritional needs, bacteria can generally replicate within the intra- or extracellular body fluids of even a malnourished host. Viral replication, on the other hand, is an intracellular event requiring a usurpation of molecular mechanisms, biochemical pathways, and substrate molecules already present within a host cell. If malnutrition causes functional derangements of host cell metabolism, viral replication may not flourish. Malnutrition could thus ameliorate the severity of some viral diseases and thereby appear antagonistic. Antiviral drugs that interfere with a key metabolic process have an analogous action in their ability to inhibit viral replication. Although parasites possess the sophisticated molecular machinery necessary for maturation and replicative cycles, their relatively large size and complex nutritional requirements may cause them to compete with host cells for available key nutrients and substrate molecules. Parasites are occasionally more successful than the host in that competition as illustrated by the development of megaloblastic anaemia in some patients with fish tapewarm infestation. Uptake of vitamin K by these parasites causes an overt deficiency in the host.
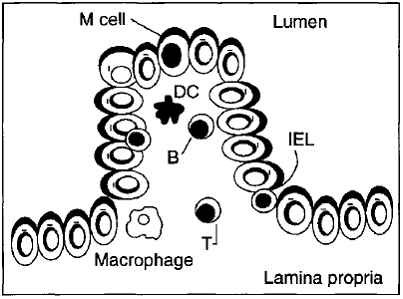
Malnutrition affects the epithelium that lines the vast mucosal surfaces of the gastrointestinal tract and enhances the possibility of infection. The existence of the M cell in the intestinal follicle-associated epithelium allows transport of enteric microorganisms, therefore several pathogenic bacteria exploit the M cell transport mechanism to infect mucosal tissues and/or spread systemically before they can be halted by an immune response.

Fig. Diagram of an M cell that allows the transport of the antigen across the epithelial barrier.
The interactions of microorganisms with M cells, especially Vibrio cholerae, Salmonella typbimurium, Shigella flexneri and reovirus were reported. The nutritional status of the host can have important effects on the final adequacy of host defensive measures and the outcome of an infection.
Imume system behaviour under malnutrition
The development, maintenance and optimal functioning of the immune system depend on balanced and adequate nutrition. Both deficiency and excess of a number of nutrients adversely affect the number and activity of immune cells. In this sense, many metabolic pathways require specific nutrients as cofactors. The antioxidative defence mechanisms employed by the body are also heavily dependent on the nutritional intake of the individual and involve a variety of vitamins (e.g. vitamins C and E), trace elements (e.g. zinc and selenium) and amino acids (e.g. cysteine). Multiple- rather than single-nutrient deficiencies are often the causes for a compromised immune system and an increased risk of infection, as observed in patients with protein-energy malnutrition (PEM).
In malnourished subjects anatomical changes involve atrophy of primary as well as secondary immune organs such as the thymus and spleen, respectively. Malnutrition impairs mainly cell-mediated immunity, which is altered at an early stage in the development of undernutrition. Nevertheless, some alterations in phagocytosis and the complement system have also been described in PEM. B cell function has so far not been shown to be affected by PEM to the same extent as that of T lymphocytes. In addition, the production of several cytokines, such as interleukin-1 (IL-1), interleukin-2 (IL-2), and interferon-y (IFN-y), is decreased. Moreover, malnutrition alters the ability of T lymphocytes to respond appropriately to cytokines. There is little work on the effect of malnutrition on the integrity of physical barriers, quality of mucus, or several other innate immune defences. For example, lysozyme concentrations are decreased, largely as the result of reduced production in monocytes and neutrophils and an increased excretion in urine.
Effects of micronutrients on the immune system
Several trace elements and vitamins have an essential role in key metabolic pathways of immune cells functions. Isolated deficiencies of micronutrients are rare with the exception of zinc, iron, and vitamin A. Observations in laboratory animals and findings in the rare patient with a single nutrient deficiency have confirmed the crucial role of several vitamins and trace elements in immunocompetence. Alterations in immune responses occur early in the course of reduction in micronutrient intake, and the extent of immunological impairment depends on the type of nutrient involved, its interactions with other essential nutrients, the severity of the deficiency, the presence of concomitant infection, and the age of the subject. Thus, tests of immunocompetence are useful in titration of physiological needs and in assessment of safe lower and upper limits of micronutrient intakes.
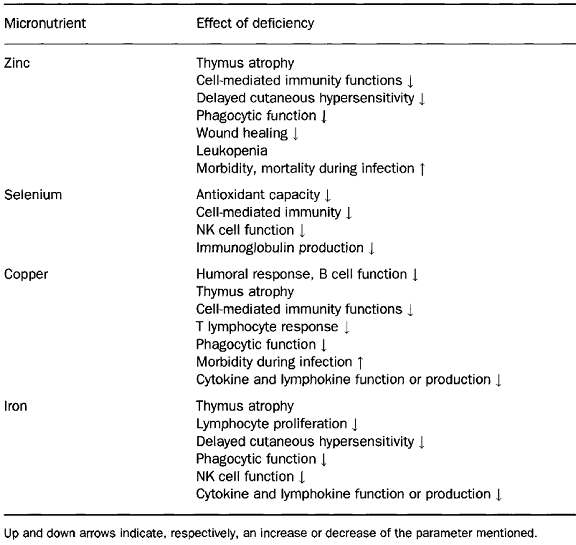
Many studies have pointed out that micronutrients such as zinc, selenium, iron, copper, carotene, vitamins A, C, and E and folic acid can influence several components of the nonspecific immunity.
Zinc
The essentiality of zinc for humans was already first documented in the 1960s. In human subjects body growth and development are strictly dependent on zinc. The nervous, reproductive and immune systems are particularly influenced by zinc deficiency, as well as by increased levels of zinc.
More than 300 metalloenzymes have been identified as being zinc (Zn) dependent. Many of these are critical for cellular metabolic pathways, including those that mediate phagocyte and lymphocyte functions. Zinc deficiency is associated with lymphoid atrophy, decreased delayed-type hypersensitivity (DTH) cutaneous responses, delayed homograft rejection, and a lower thymulin (thymic hormone) activity. It is not surprising then that zinc deficiency results in profound immunodeficiency. The salient changes observed are in
(a) phagocytes: reduced ingestion of microorganisms, impaired chemotactic migration, decreased activity of reduced oxidase, which is a cofactor for phospholipases A2 and C and instability of cell membranes possibly due to oxidation of arachidonic acid by iron complexes;
(b) cell-mediated immunity: imbalance between T helper type 1 (Thl) and Th2 functions, reduced lymphocyte proliferation response, decreased CD4 : CDS cell ratio and helper T cell function, impaired natural killer (NK) cell function, reduced thymulin activity (a thymic hormone); and
(c) humoral immunity: decreased antibody production after challenge with T cell dependent antigens and alloantigens.
A slightly excessive intake of certain nutrients such as zinc may be associated with enhanced immune responses.
The moderate deficiencies in zinc noted in sickle cell anaemia, renal disease, chronic gastrointestinal disorders, acrodermatitis enteropathica, virus-associated immunodeficiency, diarrhoeas, and in elderly persons can greatly alter host defence systems, leading to increases in opportunistic infections and mortality rates. Conversely, short periods of Zn supplementation substantially improve immune defence in individuals with these diseases.
Selenium
The trace mineral selenium is an essential nutrient of fundamental importance to human biology. As selenocysteine, the 21st amino acid, selenium (Se) is a component of selenoproteins, some of which have important enzymatic functions. In the active site selenium functions as a redox centre; the best-known example of this redox function is the reduction of hydrogen peroxide and damaging lipid and phospholipid hydroperoxides to harmless products (water and alcohols) by the family of selenium-dependent glutathione peroxidases. This function helps to maintain membrane integrity, protects prostacyclin production, and reduces the likelihood of propagation of further oxidative damage to biomolecules such as lipids, lipoproteins, and DNA with the associated increased risk of conditions such as atherosclerosis and cancer.
Selenium has additional important health effects particularly in relation to the immune response and viral disease. This trace mineral is normally found in significant amounts in immune tissues such as liver, spleen, and lymph nodes. Both cellmediated immunity and B cell function can be impaired in selenium deficiency. By way of contrast, supplementation with selenium has marked immunostimulating effects, including an enhancement of proliferation of activated cytotoxic T cells and an increased NK cell activity. These effects have been related to the ability of selenium to enhance interleukin-2 receptor (IL-2R) expression on the surface of activated lymphocytes and NK cells.
Copper
Copper is known to play an important role in the development and maintenance of the immune system but its exact mechanism of action is not yet known. The role of copper includes both B cell and T cell related deficiencies. Impaired antibody formation, inflammatory response, phagocytic killing power, and lymphocyte stimulation responses, as well as thymic atrophy, have been well documented.
Some of the previous research showed that IL-2 is reduced in copper deficiency and is likely the mechanism for T cell proliferation reduction. Similarly, the number of neutrophils in human peripheral blood and their ability to generate superoxide anion and kill ingested microorganisms is reduced in both overt and marginal copper deficiency. In addition, infections decrease both serum copper and zinc aggravating the situation and further impairing the defence system. Neutrophillike HL-60 cells accumulate copper as they differentiate into a more mature cell population and this accumulation is not reflected by increases in Cu/Zn superoxide dismutase or cytochrome-c oxidase activities. The identity of copper-binding proteins in neutrophil-like HL-60 may be useful in learning new functions of copper.
Iron
Iron deficiency is the most widespread nutrient deficiency in the world today. There is a large body of evidence accumulated from animal and human studies to indicate that iron deficiency states are associated with alterations in cellular function, growth, motor development, behaviour and cognitive function. No controversy exists about the deleterious effects of iron deficiency on immune responses; almost all published studies indicate that individuals with iron deficiency show impairment of cell-mediated immunity (DTH responses, T lymphocyte proliferation response to mitogens and antigens, production of cytokines such as IL-2 and IFN-y). In addition, phagocyte microbicidal function, NK activity and mucosal immunity are impaired. Apparently B cell and antibody formation are not affected. These alterations may well be linked to changes in the activity of iron-dependent enzymes such as myeloperoxidase and ribonucleotide reductase. In addition, physical changes in the mucosal epithelia may also be important. A more recent study indicates that iron presence can help monocytes to suppress Mycobacterium tuberculosis growth.
Iron-mediated growth suppression was correlated with selective suppression of tumour necrosis factor-a (TNF-a) release from infected monocytes and decreased monocyte sensitivity to exogenously added TNF. Transferrin is found not only in blood but also in all body fluids and is the normal mechanism for withholding iron from the infectious agent, as is lactoferrin. Conalbumin and lactoferrin have stronger iron binding properties than do most bacterial siderophores and are normally highly unsaturated. When molecules of lactoferrin become 40% saturated with iron, they are assimilated by macrophages that have been attracted to the site of infection and much of the iron is incorporated into ferritin.
Ferritin functions as an iron withholding rather than an iron-transport agent. In an iron-deficient host with reduced immune function, lack of available iron for agent replication is protective. When individuals whose resistance to infection is compromised by iron deficiency are given parenteral iron or large doses of oral iron, a disastrous exacerbation of the infection and death may occur. This happens because the agent is supplied with iron for replication before the host immune system has had time to recover. However, in field studies, supplementation of poorly nourished adults with physiological amounts of up to l00mg Fe/day and proportionately less for children, consistently results in decreased morbidity from infectious diseases.
Antioxidant vitamins
The oxidant/antioxidant balance is an important determinant of immune cell function, including maintaining integrity and functionality of membrane lipids, cellular proteins, nucleic acids, and for control of signal transduction and gene expression on immune cells. Thus, optimal function of the host defence system depends upon an adequate supply of antioxidant vitamins and, on the other hand, impaired host defence activity can act as a very early and sensitive marker of marginal deficiency of antioxidant vitamins. In humans, dietary supplementation with ascorbic acid, tocopherols and vitamin A has been shown to enhance a number of aspects of immunity and resistance to disease.
Vitamin A
Atrophy of lymphoid organs, including spleen, thymus, and lymph nodes, has been reported in vitamin A-deficient animals. However, some of these effects may be caused by loss of appetite and decreased food intake. Changes in spleen cell number are observed in the early stages of vitamin A deficiency and might be a more sensitive indicator of vitamin A deficiency, which can also affect the function of different cells of the immune system. In addition, vitamin A deficiency reduces NK activity, and is associated with a lower production of interferon.
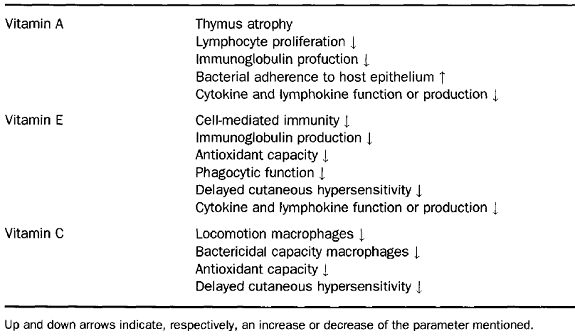
Vitamin A is essential for the maintenance of epidermal and mucosal integrity, thus low plasma vitamin A concentrations have deleterious effects on membrane integrity and mucosal function. It is not surprising that impaired antibody response to viral and parasitic antigens has also been pointed out in vitamin A deficiency in animals. It is well-known that impaired intestinal immunoglobulin A (IgA) production is attributed to impairment of gut-associated immune response. As vitamin A deficiency has been associated with an increased morbidity and mortality from infectious diseases (diarrhoea and respiratory infections), several investigators have attempted to improve the immune response and, thus, resistance to infection by vitamin A supplementation in vitamin A-deficient subjects.
Vitamin E
Vitamin E is the major peroxidation chain-breaking antioxidant in membranes. Membrane phospholipids of immunocompetent cells have a high content of polyunsaturated fatty acids and are prime targets for free radical reactions. Release of reactive oxygen species by phagocytes on encountering pathogens and rapid lymphocyte proliferation following antigenic stimulation expose the immune cells to high levels of oxidative stress. Thus, it is not surprising that cells of the immune system have higher vitamin E content than other cells of the body. Both deficiency and supplementation of vitamin E have been shown to alter the immune response and resistance against infection. The influence of vitamin E on the immune function has been shown to affect different aspects of immune functions including T cell response, antibody production, NK cell activity, phagocytic activity, and the production of immunoregulatory molecules. In humans, primary deficiency of vitamin E rarely occurs, whereas secondary deficiency is observed as a consequence of certain diseases such as primary cirrhosis, intestinal malabsorption disorders and several viral hepatitis and human immunodeficiency virus (HIV)-l infection. In addition, vitamin E deficiency is associated with increased infectious diseases and the incidence of tumours. In contrast, vitamin E is one of the few nutrients for which supplementation at higher than established dietary requirements has been shown to enhance immune response and resistance to disease and this seems to be especially important in the aged. The beneficial effects of supplementation on the host immune system include enhanced humoral and cell-mediated immunity and increased efficacy of phagocytosis in humans.
Vitamin C
Vitamin C appears to affect most aspects of the immune system. It is found in high concentrations in leukocytes, it is rapidly utilized during infection, and reduced plasma levels are often associated with reduced immune function. However, the belief that high intakes of vitamin C will prevent the onset of common cold has not been scientifically substantiated. A decrease in the duration of cold episodes and the severity of symptoms seem more plausible, although the benefits that have been observed in different studies show a large variation. The explanation for this amelioration of symptoms could be derived from the decrease in the inflammatory effects due to the reaction of vitamin C with the phagocyte derived oxidants released extracellularly during infection episodes, thus being neutralized. In a placebo-controlled, double-blind intervention study, responses to DTH skin tests were significantly reduced in healthy men submitted to a vitamin C-deficient diet for 60 days. This study showed that moderate vitamin deficiency reduces cellmediated immunity. On the other hand, supplementation has been shown to increase neutrophil motility and mitogen-stimulated lymphocyte proliferation in young males as well as decrease the incidence of post-race infections in marathon runners.
Vitamin C is one of the food components with higher antioxidant properties as demonstrated, for instance, on the protection of lipids in plasma and low-density lipoproteins (LDL) against detectable peroxidative damage. Vitamin C also serves as an electron donor to vitamin E radicals generated in the cell membrane during oxidative stress. Some effects of vitamin C, such as the decrease in DNA damage by reactive oxygen species after supplementation, still remain to be established in humans. However, their function as antioxidant may be associated to atherosclerosis and cancer prevention. The effect of simultaneous supplementation with several vitamins over the immune system has been addressed many times. A significant decrease in the number of sick days and in the use of antibiotics, as well as an increased antibody response to the flu vaccine, in a group of healthy elderly subjects who supplemented their diets with a multivitamin supplement that contained 100% of the recommended daily allowance (RDA) of most vitamins and moderately higher amounts of antioxidant vitamin C (80 mg/day), vitamin E (44 mg/day), and (3-carotene (I6mg/day) was reported. The importance of maintaining adequate amounts of antioxidants throughout life, is based on a potential long-term effect of prevention of the accumulative damage caused by reactive oxygen species being made manifest in later years.